 |
 |
- Search
| Intest Res > Epub ahead of print |
|
Abstract
Background/Aims
Methods
Results
Conclusions
ADDITIONAL INFORMATION
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
Shiga H received lecture fees from Mitsubishi Tanabe Pharma Corp., AbbVie Inc., EA Pharma Co. Ltd., Janssen Pharmaceutical K.K., Takeda Pharmaceutical Co. Ltd., Pfizer Inc., Gilead Sciences Inc., and Mochida Pharmaceutical Co. Ltd. Kakuta Y received research grants from AbbVie Inc., Daiichi Sankyo Co. Ltd., Kyowa Kirin Co. Ltd. PRECISION IBD, and Janssen Pharmaceutical K.K., and received lecture fees from Mitsubishi Tanabe Pharma Corp., and Janssen Pharmaceutical K.K. Masamune A received research grants from Zeria Pharmaceutical Co. Ltd., JIMRO Co. Ltd., Mochida Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Corp., EA Pharma Co. Ltd., and Takeda Pharmaceutical Co. Ltd. and received lecture fees from EA Pharma Co. Ltd. and Takeda Pharmaceutical Co. Ltd. The remaining authors declare no conflicts of interest.
Data Availability Statement
The data underlying this article cannot be shared publicly given the privacy of the individuals who participated in the study. The data will be shared on reasonable request to the corresponding author.
Author Contributions
Conceptualization: Shiga H. Data curation: Shiga H, Nagai H, Shimoyama Y, Naito T, Moroi R. Formal analysis: Shiga H. Investigation: Shiga H. Methodology: Shiga H. Supervision: Kakuta Y, Kinouchi Y, Masamune A. Writing - original draft: Shiga H. Writing - review & editing: Shiga H, Kakuta Y, Kinouchi Y, Masamune A. Approval of final manuscript: all authors.
Fig.┬Ā1.
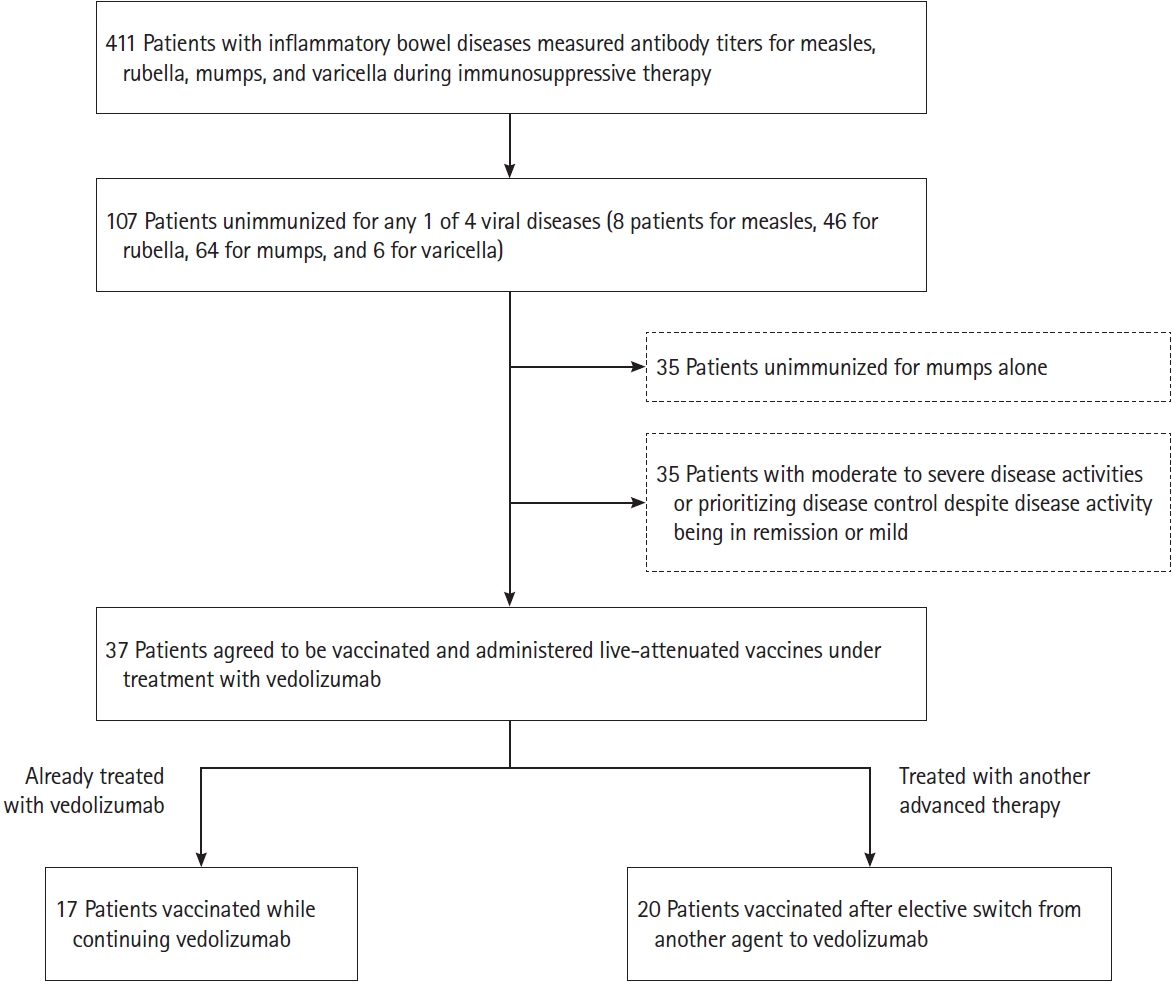
Fig.┬Ā2.
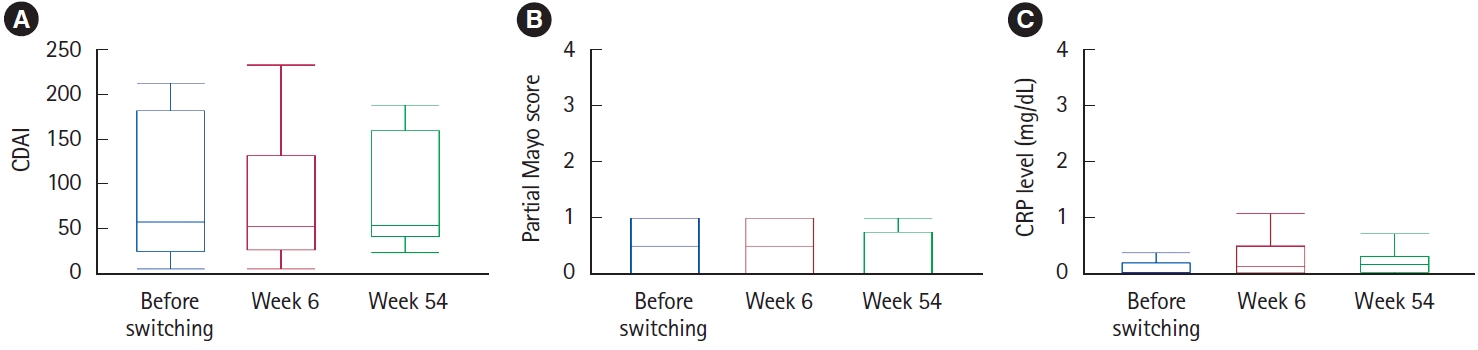
Fig.┬Ā3.
Table┬Ā1.
| Characteristic | Originally receiving VDZ (n = 17) | Switched to VDZ (n = 20) | Total (n = 37) |
|---|---|---|---|
| Sex | |||
| ŌĆāMale | 14 (82.4) | 15 (75.0) | 29 (78.4) |
| ŌĆāFemale | 3 (17.6) | 5 (25.0) | 8 (21.6) |
| Age (yr) | |||
| ŌĆāAt diagnosis | 27 (21.0-32.5) | 23 (18.0-27.0) | 23 (20.0-29.0) |
| ŌĆāAt enrollment | 30 (22.5-35.0) | 35 (27.0-41.5) | 33 (23.5-38.5) |
| Disease duration (yr) | 1 (0.0-4.0) | 10 (5.5-15.0) | 6 (0.5-10.0) |
| Disease type | |||
| ŌĆāCD | 7 (41.2) | 16 (80.0) | 23 (57.2) |
| ŌĆāŌĆāRemissiona | 6 | 13 | 19 |
| ŌĆāŌĆāMild severitya | 1 | 3 | 4 |
| ŌĆāUC | 10 (58.8) | 4 (20.0) | 14 (42.8) |
| ŌĆāŌĆāRemissiona | 8 | 4 | 12 |
| ŌĆāŌĆāMild severitya | 2 | 0 | 2 |
| Disease location in CD | |||
| ŌĆāIleitis type | 4 (57.1) | 2 (12.5) | 6 (26.1) |
| ŌĆāIleocolitis type | 1 (14.3) | 11 (68.8) | 12 (52.2) |
| ŌĆāColitis type | 2 (28.6) | 3 (18.7) | 5 (21.7) |
| Disease extent in UC | |||
| ŌĆāTotal colitis type | 6 (60.0) | 3 (75.0) | 9 (64.3) |
| ŌĆāLeft-sided colitis type | 4 (40.0) | 1 (25.0) | 5 (35.7) |
| Smoking habit | |||
| ŌĆāCurrent or past smoker | 9 (52.9) | 11 (55.0) | 132 (32.3) |
| ŌĆāNever | 8 (47.1) | 9 (45.0) | 277 (67.7) |
| Biological agents used | |||
| ŌĆāIFX | 0 | 10 (50.0) | |
| ŌĆāADA | 0 | 7 (35.0) | |
| ŌĆāGLM | 0 | 1 (5.0) | |
| ŌĆāUST | 0 | 2 (10.0) | |
| ŌĆāVDZ | 17 (100.0) | 0 | |
| Thiopurines used | |||
| ŌĆāPresent | 4 (23.5) | 13 (65.0) | 17 (45.9) |
| ŌĆāŌĆāAzathioprine | 2 | 10 | 12 |
| ŌĆāŌĆā6-Mercaptopurine | 2 | 3 | 5 |
| ŌĆāAbsent | 13 (76.5) | 7 (35.0) | 20 (54.1) |








