 |
 |
- Search
| Intest Res > Volume 21(3); 2023 > Article |
|
Abstract
Background/Aims
The data on the prevalence of hepatitis B virus (HBV) and hepatitis C virus (HCV) infection in patients with inflammatory bowel disease (IBD) are conflicting. The present systematic review was thus conducted to study the prevalence of HBV and HCV markers in patients with IBD.
Methods
A comprehensive literature search of 3 databases was conducted from 2000 to April 2022 for studies evaluating the prevalence of HBV or HCV in patients with IBD. Pooled prevalence rates across studies were expressed with summative statistics.
Results
A total of 34 studies were included in the final analysis. The pooled prevalence of hepatitis B surface antigen (HBsAg) and hepatitis B core antibodies were 3.3% and 14.2%, respectively. In HBsAg positive IBD patients, hepatitis B e antigen positivity and detectable HBV DNA were seen in 15.3% and 61.0% of patients, respectively. Only 35.6% of the IBD patients had effective HBV vaccination. The pooled prevalence of anti-HCV and detectable HCV RNA were 1.8% and 0.8%, respectively. The pooled prevalence of markers of HBV infection was higher in Asian studies, while the prevalence of markers of HCV infection was higher in European studies. The prevalence of viral hepatitis markers was similar between IBD patients and the general population and that between ulcerative colitis and Crohn’s disease.
Conclusions
The prevalence of markers of viral hepatitis remains same as the general population with significant regional variations, although the quality of evidence remains low due to publication bias. Only a small proportion of IBD patients had an effective HBV vaccination, requiring improvement in screening and vaccination practices.
Inflammatory bowel disease (IBD), which encompasses 2 clinical forms, namely ulcerative colitis (UC) and Crohn’s disease (CD), is a heterogeneous group of inflammatory disorders of the gastrointestinal tract [1]. Though the disease is more prevalent in the West, there has been an increasing incidence in Asian countries in the last two decades [2,3]. The treatment of IBD primarily involves immunosuppressive and immunomodulatory drugs. This not only increases the chance of prevalence of various chronic infective diseases like chronic hepatitis C virus (HCV) and hepatitis B virus (HBV) but also may lead to reactivation of the latter disease [4-6]. This will have more impact on Asian countries due to the moderately high prevalence of HBV infection [7]. Therefore, screening for chronic HBV and HCV is crucial before starting the immunosuppressive treatment in IBD. Nonalcoholic fatty liver disease is becoming more common in patients with IBD [8]. On the other hand, the drugs like thiopurines may provoke liver damage even in the normal liver or may increase the viremia in chronic hepatitis C, leading to the progression of liver fibrosis [9]. Therefore, to prevent the progression of liver disease due to the interplay in the management of IBD and viral hepatitis, identification of viral hepatitis is important in while treating IBD [10].
Although there are scarce case-control data on prevalence of chronic viral infections in IBD patients, the prevalence is thought to be similar to the general population [11]. The European Crohn’s and Colitis Organisation guideline recommends the measurement of IgG antibodies against HBV, and HCV for all IBD patients, either at the initial disease diagnosis or while starting treatment with immunosuppressive agents [12]. There is large data on overall prevalence of HBV and HCV infection among general population. However, to the best of our knowledge, there is hardly any previously published systematic review or meta-analysis on prevalence among IBD patients. The main objective of this meta-analysis was to evaluate the prevalence of HBV and HCV infection in patients with IBD.
The present systematic review and meta-analysis were conducted as per the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) [13] and the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [14] guidelines. Institutional review board approval and informed patient consent were not applicable for systematic review and meta-analysis.
Electronic databases of MEDLINE, Embase, and Science Direct were searched from 2000 to April 2022 for the title and abstracts of all relevant studies using the keywords: (IBD or “Inflammatory bowel disease” or “Crohn’s disease” or CD or “Ulcerative colitis” or UC) and (Hepatitis B or HBV or Hepatitis C or HCV). Two independent reviewers (S.G. and S.A) screened the title and abstract of the retrieved studies and assessed the full texts for eligibility before including them. The bibliographies of the included studies were searched for any relevant studies. A third reviewer (A.K.) resolved any disagreement.
Studies included in this meta-analysis were prospective or retrospective studies fulfilling the following criteria: (1) Study population-patients with IBD; (2) Diagnostic test-markers of HBV infection (hepatitis B surface antigen [HBsAg], hepatitis B core antibody [anti-HBc], hepatitis B e antigen [HBeAg], and HBV DNA), markers of immunity against HBV (anti-HBs), and markers of HCV infection (anti-HCV and HCV RNA); and (3) Outcomes-seroprevalence of HBV and HCV, effective immunization. Conference abstracts, case series, review articles, correspondences, and editorials were excluded.
Data were entered into a structured data extraction form with the following parameters: first author, year of publication, location of study, number of patients, study population description, risk factors for viral hepatitis, history of vaccination, and serological markers. The quality of the included studies was assessed by two reviewers (S.G. and S.K.) using the Joanna Briggs Institute (JBI) critical appraisal tools for use in systematic reviews (Supplementary table 1) [15]. JBI appraisal for incidence/prevalence data includes questions about the appropriateness of study sample and selection, description of setting and subjects, completeness of provided data and analysis, and the appropriateness of measuring the condition. The quality of study was determined as per the score (high: 7-9, medium: 4-6, and low: < 4). A third independent individual (A.K.) was consulted to determine the best score based on any discrepancy in the study quality assessment.
The pooled proportions were computed using a random-effect method with an inverse variance approach [16]. Prior to statistical analysis, a continuity correction of 0.5 was applied when the incidence of an outcome was zero in a study. Dichotomous variables were analyzed using the odds ratio (OR) and MantelHaenszel test. The heterogeneity was assessed by I2 and the p-value of heterogeneity. A P-value of < 0.10 was taken as statistically significant while I2 values of 25%, 50%, and 75% were considered as cutoffs for low, moderate, and considerable heterogeneity, respectively [17]. The assessment of publication bias was done by evaluating the asymmetry of the funnel plot and quantified using Egger’s test. Sensitivity analysis was performed by analyzing prevalence data based on continent and study design and by leave-one-out meta-analysis. Meta-regression was used to explore heterogeneity induced by the relationship between moderators and study effect sizes. All statistical analyses were performed using RevMan version 5.4 and STATA software version 17 (StataCorp., College Station, TX, USA).
The search strategy yielded 2,194 records from which 1,267 studies were screened after removal of duplicates. Fig. 1 shows the flow chart for study selection and inclusion process. A total of 34 studies [18-51] were included in the final analysis. Table 1 shows the characteristics of the included studies. Among these, 17 studies were prospective [18-25,30,31,34,39,45,48-51] and 17 were retrospective in nature [26-29,32,33,35-38,40-44,46,47]. The majority of the studies were from Europe [18,20,24-27,31,34,36,39,40,47,49] and Asia [28,30,33,35,37,38,41,42,45,46,51]. The number of patients in the studies varied from 74 to 5,096 with a mean age from 32.9 to 50.8 years. Majority of the studies included consecutive patients with IBD while 7 studies [20,29,31,34,36,40,44] analyzed data of patients being planned for biologicals. Prior risk factors for viral hepatitis and vaccination history were reported in 9 studies [21,25,26,30,31,33,38,43,51] and 10 studies [25,30,31,33,34,37,39,41,45,49], respectively. The study quality assessment is summarized in Supplementary Table 1. Among the included studies, 22 studies were of high quality while 12 studies were of medium quality.
A total of 30 studies [19-21,23-43,44-51] with 17,022 patients reported on HBsAg positivity in patients with IBD. The pooled prevalence of HBsAg was 3.3% (95% confidence interval [CI], 2.5-4.0; I2 = 91.6%) with significant heterogeneity among the studies (Fig. 2). Fig. 3A shows the geographic heat map for HBsAg positivity in IBD patients. On subgroup analysis, the pooled prevalence of HBsAg in patients with UC and CD were 3.3% (95% CI, 2.3-4.4; I2 = 86.5%) and 2.9% (95% CI, 2.0-3.8; I2 = 88.2%) (Supplementary Figs. 1 and 2), respectively. There was neither any difference in the odds of HBsAg positivity between patients with UC and CD (OR, 1.15; 95% CI, 0.96-1.37; I2 = 0%) nor between IBD and general population (OR, 1.08; 95% CI, 0.93-1.24; I2 = 0%) (Supplementary Figs. 3 and 4).
Overall, 9 studies [19,23,24,26,31,35,36,39,47] reported on the presence of detectable HBeAg in patients with HBsAg positivity. The pooled prevalence of HBeAg positivity in HBsAg positive cases was 15.3% (95% CI, 6.9-23.7; I2 = 67.9%) (Supplementary Fig. 5). The presence of detectable HBV DNA was reported in 15 studies with 10,663 patients [21,24-26,28,31-34,38,40,41,43,50,51]. The pooled prevalence of detectable HBV DNA in patients with IBD and IBD with HBsAg positive cases were 1.0% (95% CI, 0.6-1.4; I2 = 75.0%) and 61.0% (95% CI, 42.1-79.9; I2 = 91.6%), respectively (Supplementary Fig. 6).
The prevalence of anti-HBc (with or without HBsAg) in patients with IBD was reported in 25 studies with 12,265 patients [19-21,23-25,27,29-34,37,39-41,43-45,47-51]. The pooled anti-HBc positivity in IBD patients was 14.2% (95% CI, 10.6-17.8; I2 = 98.2%), with significant heterogeneity among the studies (Supplementary Fig. 7). On subgroup analysis, the pooled anti-HBc positivity in patients with UC and CD were 20.3% (95% CI, 12.8-27.8; I2 = 98.1%) and 16.1% (95% CI, 10.0-22.1; I2 = 97.8%), respectively (Supplementary Fig. 8 and 9). Patients with IBD had a higher prevalence of anti-HBc positivity compared to controls (OR, 1.48; 95% CI, 1.02-2.13; I2 = 90%) and among patients with IBD (Supplementary Fig. 10), UC was associated with higher odds of anti-HBc positivity compared to CD (OR, 1.29; 95% CI, 1.03- 1.61; I2 = 49%) (Fig. 4).
Effective immunization was defined as the presence of antiHBs titer ≥ 10 mIU/mL without anti-HBc and HBsAg. The presence of protective antibody against HBV in patients with completed immunization was reported in 10 studies with 4,895 patients [20,23-25,27,30,31,32,36,38,39,41,46,47]. Among patients with IBD, only 35.6% (95% CI, 28.7-42.4; I2 = 96.5%) of the patients had effective vaccination (Supplementary Fig. 11).
Overall, 22 studies with 10,304 patients of IBD reported on anti-HCV prevalence [18-20,22-27,31-34,36,39-42,46-50]. The pooled prevalence of anti-HCV positivity was 1.8% (95% CI, 1.2-2.4; I2 = 82.1%) with significant heterogeneity among the studies (Fig. 5). Fig. 3B shows the geographic heat map for anti-HCV positivity in IBD patients. On subgroup analysis, the pooled prevalence of anti-HCV in patients with UC and CD were 1.4% (95% CI, 0.7-2.1; I2 = 73.3%) and 1.4% (95% CI, 0.6-2.1; I2 = 80.5%), respectively (Supplementary Fig. 12 and 13). The difference in prevalence of HCV between patients with IBD and general population was reported by 5 studies. Presence of IBD was not associated with an increased odd of HCV (OR, 1.42; 95% CI, 0.93-2.18, I2 = 0%) without any heterogeneity (Supplementary Fig. 14). Overall, 9 studies compared the HCV prevalence between patients with UC and CD. There was no difference in the odds of HCV prevalence between UC and CD (OR, 1.04; 95% CI, 0.54-1.99; I2 = 52%) with significant heterogeneity (Supplementary Fig. 15).
Overall, 12 studies with 7,447 patients reported HCV RNA positivity in patients with IBD [19,20,24,25-27,31-34,41,47,48]. The pooled prevalence of HCV RNA positivity among patients with IBD and IBD patients with positive anti-HCV were 0.8% (95% CI, 0.4-1.3; I2 = 87.9%) and 78.5% (95% CI, 64.8-92.2; I2 = 91.4%), respectively (Supplementary Fig. 16).
Significant publication bias for all the outcomes except for the comparison of prevalence of HBV and HCV markers between IBD and general population and patients with UC and CD (Supplementary Fig. 17). On leave-one-out meta-analysis, there was no difference in anti-HBc positivity between UC and CD with the exclusion of the study by Tolentino et al. [21], Kim et al. [30], and He et al. [37]. Similarly, with the exclusion of studies one at time, there was no difference in the anti-HBc positivity between IBD and controls, except for the study by Kim et al. [30] Concerning HCV viremic status, HCV RNA positivity rate reduced to 0.5% (0.2-0.8) with the exclusion of the study by Morisco et al. [27].
Meta-regression analysis was conducted to assess for the source of heterogeneity for various outcomes. For HBsAg positivity and anti-HBc positivity, difference in the continent of study was a significant contributor to heterogeneity (Supplementary Fig. 18). For anti-HCV positivity, the continent of study (P= 0.016), publication year (P= 0.011) and mean age (P= 0.004) of the study population were significant covariates contributing to heterogeneity (Fig. 6).
Table 2 summarizes the pooled events rates with sensitivity analysis based on etiology, study design and continent of study.
The present analysis provides updated data on the epidemiology of HBV and HCV infection among IBD patients globally. The pooled prevalence of HBsAg in patients with IBD was 3.3% (2.5-4.2), while HBeAg positivity and detectable HBV DNA were seen in 15.3% (6.9-23.7) and 61.0% (42.1-79.9) of the HBsAg positive patients, respectively. The pooled prevalence of anti-HBc in IBD patients was 14.2% (10.6-17.8), while effective HBV vaccination was seen in only 35.6% (28.7-42.4) of the patients. The pooled prevalence of anti-HCV and detectable HCV RNA were 1.8% (1.2-2.4) and 0.8% (0.4-1.3), respectively. The odds of prevalence of HBsAg (OR, 1.08; 95% CI, 0.93-1.24) and anti-HCV (OR, 1.42; 95% CI, 0.93-2.18) were similar between IBD patients and the general population. Similarly, both patients with UC and CD had a comparable prevalence of HBsAg (OR, 1.15; 95% CI, 0.96-1.37) and anti-HCV (OR, 1.04; 95% CI, 0.54-1.99). Although the prevalence of anti-HBc was higher in patients with IBD compared to controls (OR, 1.48; 95% CI, 1.02-2.13) and in patients with UC compared to CD (OR, 1.29; 95% CI, 1.03-1.61), the odds were comparable on sensitivity analysis.
The reported global prevalence of HBsAg in 2016 was 3.9% (3.4-4.6) [52], which is similar to the HBsAg prevalence rate of 3.3% (2.5-4.0) among IBD patients in the present analysis. HBV infection may be particularly significant for patients with IBD. Firstly, IBD is no more the disease of the West, with incidence and prevalence increasing across developing countries where HBV infection is more prevalent [53]. This would imply that many patients with IBD may be exposed and infected with HBV. Secondly, HBV vaccination rates are considerably lower in developing countries, especially amongst the IBD population, which puts them at increased risk of HBV infection [54]. Finally, the immunodeficiency state acquired through immunomodulatory drugs like steroids, thiopurines, biologics, or biosimilars renders patients with IBD more vulnerable to viral reactivation, characterized by viremia with or without clinical manifestations, including fulminant life-threatening hepatitis.
In the study by Loras et al. [55], 36% (9/25) of the HBsAg positive IBD patients on immunosuppression developed reactivation, out of which 6 patients (6/9, 75%) developed hepatic failure. Treatment with ≥ 2 immunosuppressants was an independent predictor of HBV reactivation, while prophylactic antiviral therapy was protective against reactivation. Interestingly, none of the patients with isolated anti-HBc positivity developed HBV reactivation. The study by Park et al. [28] reported liver dysfunction in 25.7% of the HBsAg positive compared to 2.8% of the HBsAg-negative patients receiving immunosuppressive therapy. Lee et al. [56] reported that the liver dysfunction due to viral reactivation was 7.3% after a median time interval of 32.4 months after anti-tumor necrosis factor (anti-TNF) in IBD patients with HBV infection. The proportion of liver dysfunction was significantly higher in the non-prophylaxis group (26% vs. 8%, P= 0.02). The pooled proportion of anti-HBc positivity (present or past HBV infection) was 14.2% (10.6-17.8). This subset of patients has a moderate risk of HBV reactivation with the use of anti-TNF therapy, anti-integrin therapy or moderate to high-dose corticosteroids [6]. Therefore, despite having a similar prevalence as the general population, the risk of reactivation or liver dysfunction remains high, which could be prevented by early detection and treatment. For this reason, both ECCO and BSG guidelines recommend that all IBD patients should be tested for HBsAg, anti-HBs, and anti-HBc, preferably at the time of diagnosis [12,57].
Immunomodulators and immunosuppressants reduce the effective HBV vaccination response [12]. The study by Kim et al. [30] compared HBV markers of IBD patients with age- and sexmatched controls and reported a lower anti-HBs positivity rate (61.8% vs. 73.3%, P< 0.001) and effective vaccination in patients with IBD (38.1% vs. 44.4%, P= 0.04). They reported that around one-third of the IBD patients were susceptible to HBV and age < 30 years was a risk factor for nonimmune status in the multivariate analysis. Subsequent studies by Papa et al. [31] and Huang et al. [33] reported a similar lower rate of effective vaccination in patients with IBD, 23.9% and 21.6%, respectively. The present analysis showed that only around one-third of the IBD patients had effective vaccination and this rate was still lower for Asian studies compared to European studies (29.2% [95% CI, 22.4-36.0] vs. 42.2% [95% CI 29.3-55.1]). In a recent meta-analysis, the pooled OR of HBV response in IBD patients was lower compared to controls (OR, 0.13; 95% CI, 0.05-0.33), with pooled proportion of effective immune response being 39.7% (95% CI, 30.7-49.5) [58].
In the study by Morisco et al. [27] of the 5,096 patients with IBD, only 30.5% and 29.7% patients were investigated for HBV and HCV markers, respectively. Similarly, Vaughn et al. [29] reported that only 25% of the IBD were screened for hepatitis B in the year prior to an anti-TNF being initiated. In a survey from Australia, only 61.3% and 27% of the gastroenterologists screened their patients for HBV infection prior to anti-TNF therapy and corticosteroids, respectively [59]. In a subsequent study from France, 91% of the gastroenterologists screened IBD patients for HBV while only 46% recommended HBV vaccination for seronegative patients [60]. Thus, there is considerable uncertainty and disagreement with respect to screening and vaccination practice in IBD patients and this needs to be improved.
Concerning the variation in the prevalence of HBV across various regions, a previous analysis showed a higher prevalence of HBsAg positive population in the Western Pacific (5.7%; 95% CI, 5.1-6.6) and South-East Asian region (3.5%; 95% CI, 2.9-4.0) compared to European region (1.6%; 95% CI, 1.1-2.1) [52]. The present meta-analysis also showed that Asian studies had a higher pooled prevalence of HBsAg (5.8% [95% CI, 4.2-7.5] vs. 1.2% [95% CI, 0.8-1.6]) and anti-HBc (29.7% [95% CI, 22.1-37.3] vs. 7.5% [95% CI 5.2-9.7]) in the IBD patients compared to European studies.
The global prevalence of viremic HCV infection (HCV RNA-positive cases) for the year 2020 was reported as 0.7% (95% uncertainty interval, 0.7-0.9), which had decreased from the prevalence rate of 0.9% (0.8-1.0) for the year 2015 [61]. The present analysis also showed a similar prevalence of viremic HCV infection (0.8%; 95% CI, 0.4-1.3). Patients with HCV infection who receive immunosuppressive treatment for IBD raise several interesting concerns. Prednisone may negatively affect HCV infection by increasing the viral load. On the other hand, anti-TNF-α in IBD may not lead to reactivation of hepatitis C. Morisco et al. [27] and Loras et al. [55] reported liver dysfunction in 1 out of 10 (10%) and 8 out of 51 (15.7%) of HCV RNA positive patients, respectively. Thus, IBD patients with HCV viremia should be evaluated and treated actively to prevent hepatic dysfunction.
In a previous meta-analysis, the prevalence of anti-HCV was higher in the Asian studies compared to European studies (2.8% vs. 1.8%), but the viremic rate was higher in the Europeans (72.4% vs. 64.4%) [62]. On the contrary, the present analysis showed a significantly higher anti-HCV positivity (2.1% [95% CI, 1.3-2.9] vs. 0.6% [95% CI, 0.1-1.0]) and viremic rate (1.1% [95% CI, 0.4-1.8] vs. 0.2% [95% CI, 0.0-0.5]) in European studies compared to Asian studies. This may be due to the fact that the prevalence of HCV is higher in central Asia, while the studies included in the present meta-analysis were mostly from east, south, and south-east Asia, where the prevalence remains lower [61,62]. One interesting finding from the current meta-analysis was the reduction in the effect size of anti-HCV prevalence with publication year (Fig. 6). This decreasing prevalence of HCV in IBD patients suggests that preventative measures such as blood transfusion safety programs, single-use materials, and better aseptic perioperative rules have been effective and explains the diminishing risk for HCV.
One major limitation of this study was the significant heterogeneity between the included studies. Second, the number of primary studies outside of Asia and Europe was small, and that comparisons with other regions were not possible. It is also a concern that the number of included primary studies may affect the results, since different countries in Europe have different prevalence rates due to differences in vaccination policies [63]. Third, the data on HBV DNA or HCV RNA were unavailable in most studies. Fourth, the prevalence of chronic hepatitis B and C may be warranted in the subclassified group by age, location, and severity. However, unfortunately, no such data on the prevalence in different age groups were available in the included studies. This study estimated the pooled prevalence of hepatitis B and C among the entire IBD participants irrespective of age. So, it is crucial in future prevalence studies to consider prevalence stratification regarding age and other disease variables. Lastly, most studies did not have data on prior treatment history, risk factors, and vaccination.
Nevertheless, this is the first meta-analysis utilizing data globally to evaluate the prevalence of chronic hepatitis B and C markers in IBD patients. The current evidence suggests that the cumulative prevalence of HBV and HCV in IBD patients is sizeable and parallels the national trends in each country. Physicians should be sensitized to implement guidelines’ recommendations in clinical practice to ensure homogeneous screening, prevention, and management of chronic viral hepatitis infection in IBD patients. Further prospective, multicentric and multinational studies are required to understand the actual burden of viral hepatitis in IBD to inform the best possible public health measures and save the direct and indirect costs associated with it.
ADDITIONAL INFORMATION
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Author Contribution
Conceptualization: Giri S. Methodology: Giri S, Agrawal D, Afzalpurkar S, Kasturi S, Gopan A. Formal analysis: Giri S, Kasturi S, Gopan A. Project administration: Giri S, Sundaram S, Kale A. Visualization: Giri S. Writing-original draft: Giri S, Agrawal D, Afzalpurkar S. Writing-review and editing: Giri S, Agrawal D, Afzalpurkar S, Sundaram S, Kale A. Approval of final manuscript: all authors.
Supplementary Material
Supplementary materials are available at the Intestinal Research website (https://www.irjournal.org).
Supplementary Table 1. Study Quality Assessment Using Joanna Briggs Institute Critical Appraisal Tool
ir-2022-00094-Supplementary-Table-1.pdf
Supplementary Fig. 1. Forest for prevalence of hepatitis B surface antigen in ulcerative colitis. DL, DerSimonian and Laird method; CI, confidence interval.
ir-2022-00094-Supplementary-Fig-1.pdf
Supplementary Fig. 2. Forest for prevalence of hepatitis B surface antigen in Crohn’s disease. DL, DerSimonian and Laird method; CI, confidence interval.
ir-2022-00094-Supplementary-Fig-2.pdf
Supplementary Fig. 3. Forest plot comparing the prevalence of hepatitis B surface antigen in inflammatory bowel disease (IBD) versus controls. M-H, Mantel-Haenszel; CI, confidence interval.
ir-2022-00094-Supplementary-Fig-3.pdf
Supplementary Fig. 4. Forest plot comparing the prevalence of hepatitis B surface antigen in ulcerative colitis (UC) versus Crohn’s disease (CD). M-H, Mantel-Haenszel; CI, confidence interval.
ir-2022-00094-Supplementary-Fig-4.pdf
Supplementary Fig. 5. Forest plot for prevalence of hepatitis B e antigen positivity in hepatitis B surface antigen positive patients. DL, DerSimonian and Laird method; CI, confidence interval.
ir-2022-00094-Supplementary-Fig-5.pdf
Supplementary Fig. 6. Forest plot for prevalence of detectable hepatitis B virus DNA in hepatitis B surface antigen positive. DL, DerSimonian and Laird method; CI, confidence interval.
ir-2022-00094-Supplementary-Fig-6.pdf
Supplementary Fig. 7. Forest plot showing the pooled prevalence of hepatitis B core antibody in patients with inflammatory bowel disease with subgroup analysis based on the continent of study. DL, DerSimonian and Laird method; CI, confidence interval.
ir-2022-00094-Supplementary-Fig-7.pdf
Supplementary Fig. 8. Forest plot for prevalence of hepatitis B core antibody in ulcerative colitis. DL, DerSimonian and Laird method; CI, confidence interval.
ir-2022-00094-Supplementary-Fig-8.pdf
Supplementary Fig. 9. Forest plot for prevalence of hepatitis B core antibody in Crohn’s disease. DL, DerSimonian and Laird method; CI, confidence interval.
ir-2022-00094-Supplementary-Fig-9.pdf
Supplementary Fig. 10. Forest plot comparing prevalence of hepatitis B core antibody positivity in inflammatory bowel disease (IBD) versus controls. CI, confidence interval.
ir-2022-00094-Supplementary-Fig-10.pdf
Supplementary Fig. 11. Forest plot for effective vaccination against hepatitis B virus in inflammatory bowel disease. DL, DerSimonian and Laird method; CI, confidence interval.
ir-2022-00094-Supplementary-Fig-11.pdf
Supplementary Fig. 12. Forest plot for prevalence of anti-hepatitis C virus in ulcerative colitis. DL, DerSimonian and Laird method; CI, confidence interval.
ir-2022-00094-Supplementary-Fig-12.pdf
Supplementary Fig. 13. Forest plot for prevalence of anti-hepatitis C virus in Crohn’s disease. DL, DerSimonian and Laird method; CI, confidence interval.
ir-2022-00094-Supplementary-Fig-13.pdf
Supplementary Fig. 14. Forest plot comparing the prevalence of anti-hepatitis C virus in inflammatory bowel disease (IBD) versus controls. M-H, Mantel-Haenszel; CI, confidence interval.
ir-2022-00094-Supplementary-Fig-14.pdf
Supplementary Fig. 15. Forest plot comparing the prevalence of anti-hepatitis C virus in ulcerative colitis (UC) versus Crohn's disease (CD). M-H, Mantel-Haenszel; CI, confidence interval.
ir-2022-00094-Supplementary-Fig-15.pdf
Supplementary Fig. 16. Forest plot for prevalence of detectable hepatitis C virus (HCV) RNA in anti-HCV positive cases. HBsAg, hepatitis B surface antigen; anti-HBc, hepatitis B core antibody; CI, confidence interval.
ir-2022-00094-Supplementary-Fig-16.pdf
Supplementary Fig. 17. Funnel plot. DL, DerSimonian and Laird method; CI, confidence interval.
ir-2022-00094-Supplementary-Fig-17.pdf
Supplementary Fig. 18. Meta-regression for source of heterogeneity for hepatitis B surface antigen (HBsAg) in inflammatory bowel disease. CI, confidence interval.
ir-2022-00094-Supplementary-Fig-18.pdf
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart showing the study screening and selection process. IBD, inflammatory bowel disease.
Fig. 2.
Forest plot showing the pooled prevalence of hepatitis B surface antigen (HBsAg) in patients with inflammatory bowel disease with subgroup analysis based on the continent of study. DL, DerSimonian and Laird method; CI, confidence interval.
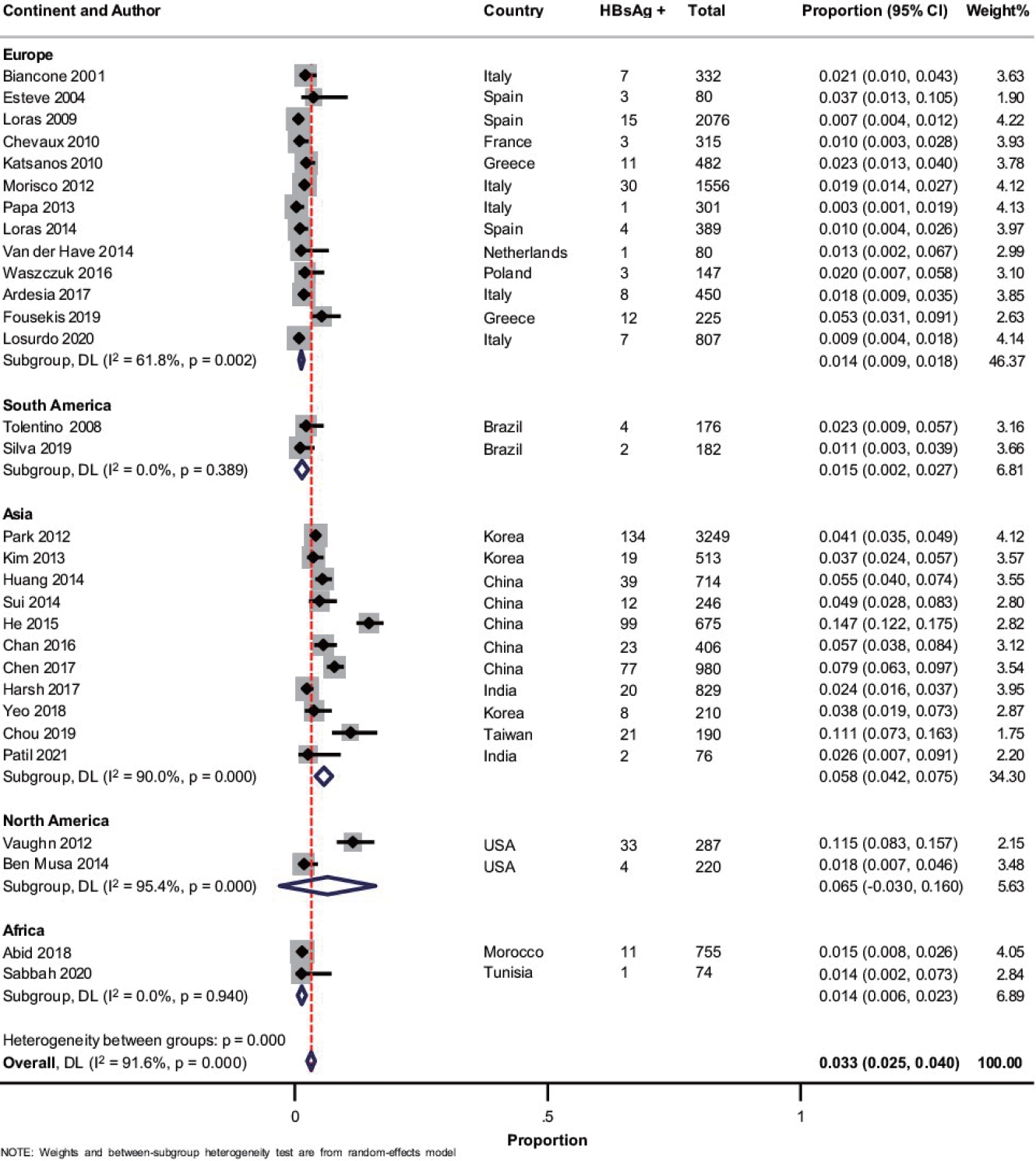
Fig. 3.
Geographic heat map for prevalence of (A) hepatitis B surface antigen and (B) anti-HCV in patients with inflammatory bowel disease. HBV, hepatitis B virus; HCV, hepatitis C virus.
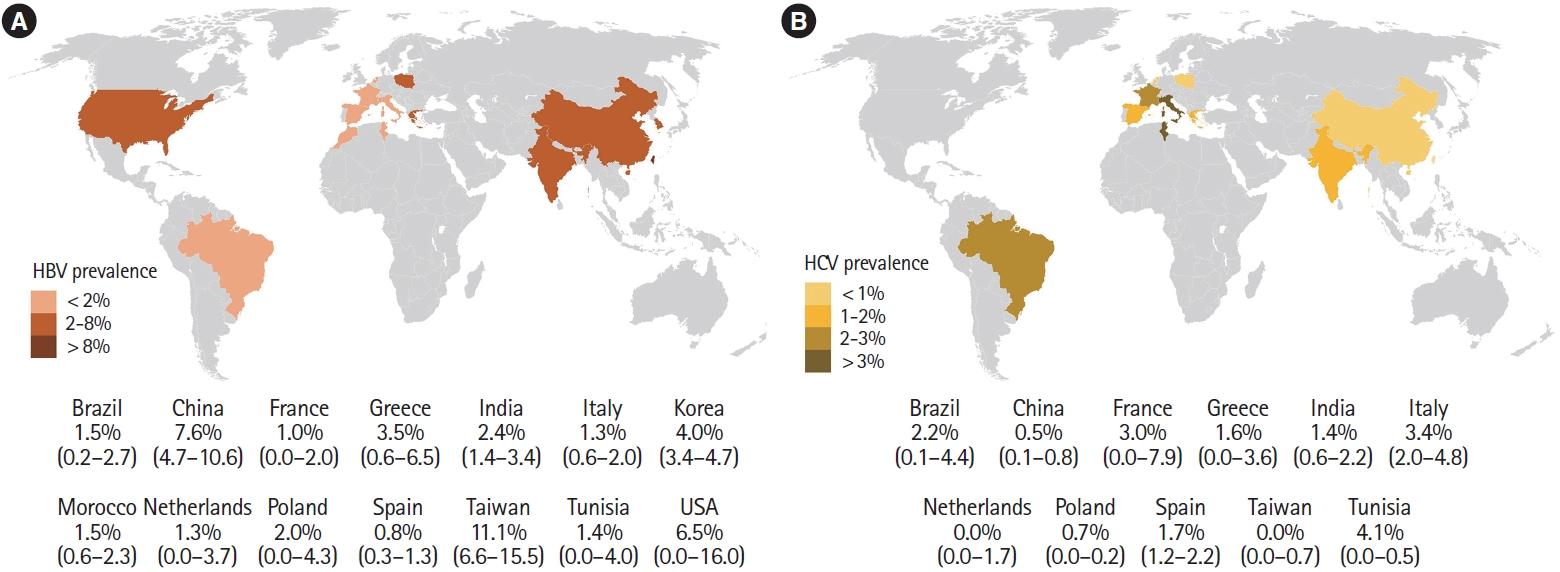
Fig. 4.
Forest plot comparing the prevalence of hepatitis B core antibody in patients with ulcerative colitis (UC) and Crohn's disease (CD). M-H, Mantel-Haenszel; CI, confidence interval.
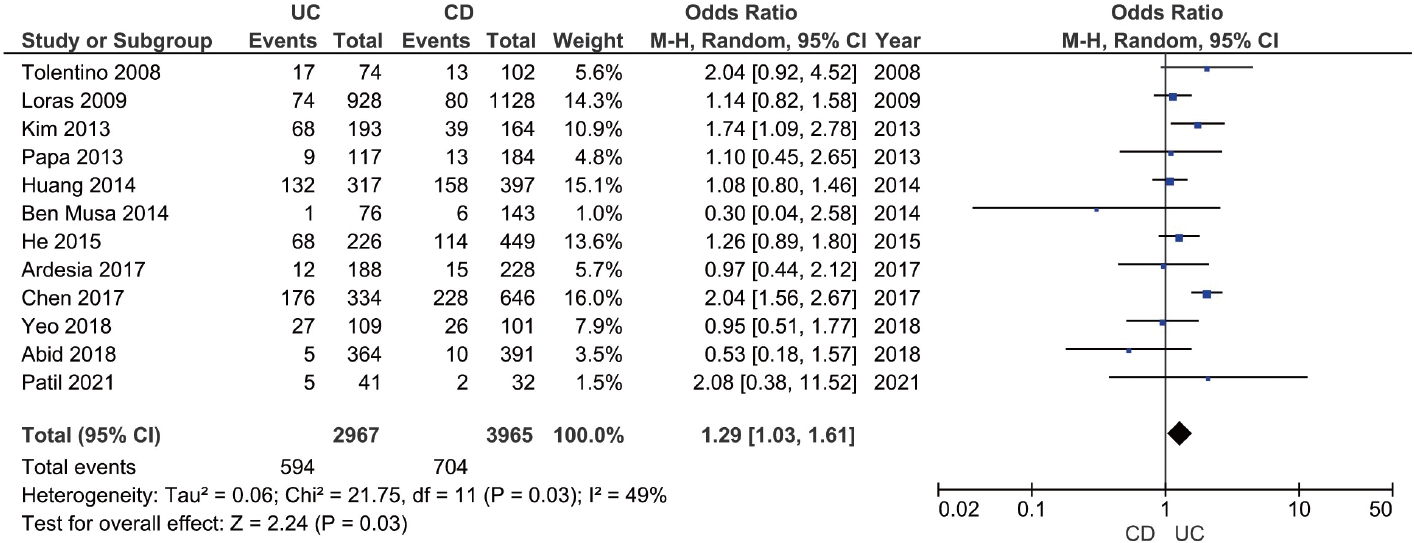
Fig. 5.
Forest plot showing the pooled prevalence of anti-hepatitis C virus (HCV) in patients with inflammatory bowel disease with subgroup analysis based on the continent of study. DL, DerSimonian and Laird method; CI, confidence interval.
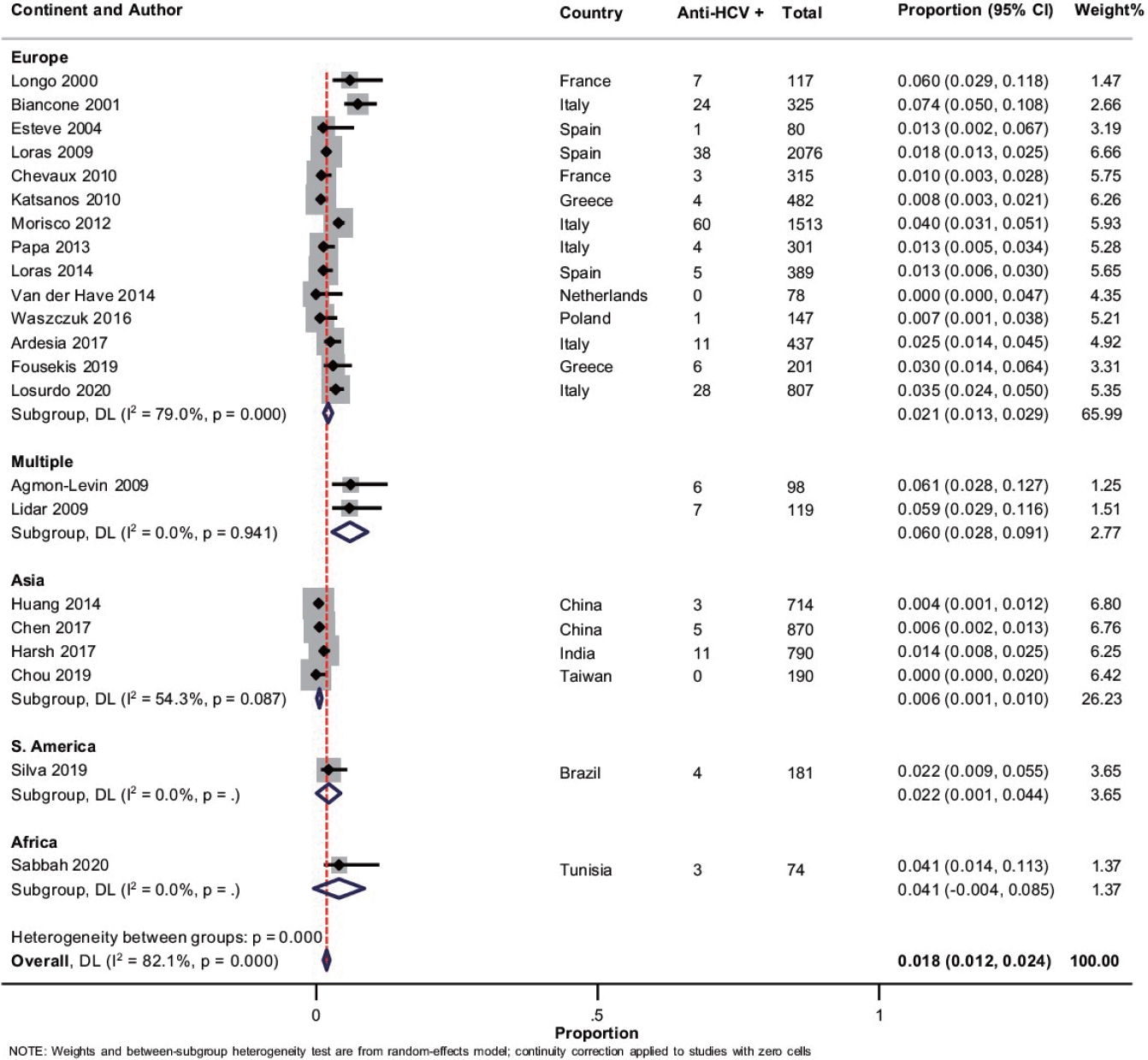
Fig. 6.
Meta-regression for the assessment of the source of heterogeneity concerning anti-hepatitis C virus (HCV) in patients with inflammatory bowel disease analyzing (A) year of publication, (B) study continent, (C) sample size, and (D) mean age. CI, confidence interval.
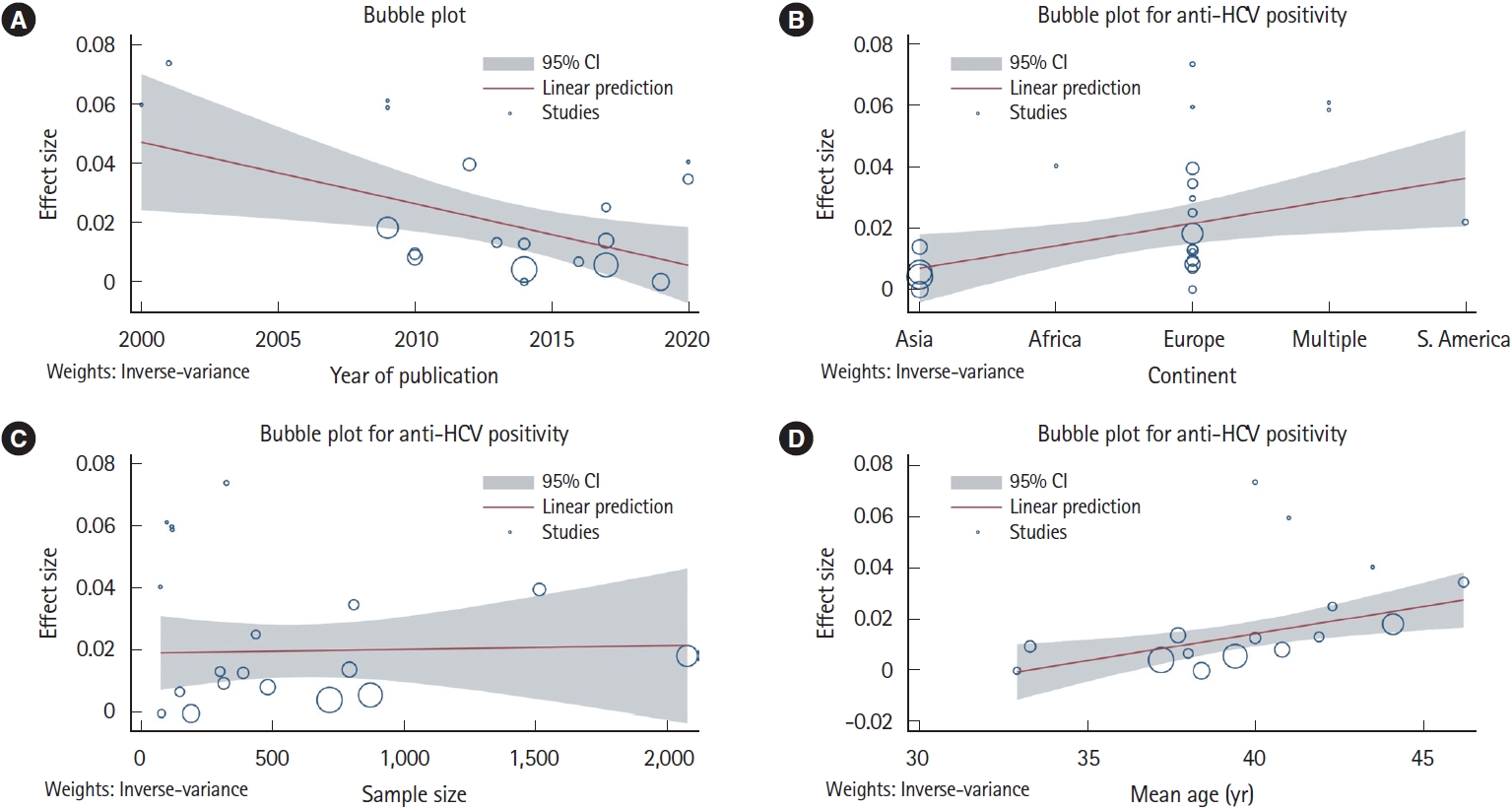
Table 1.
Characteristics of Included Studies in the Meta-Analysis
| Author (year) | Country | Study design | No. of patients | M/F | Age (yr), mean±SD | Patient selection | Risk factors (S/B) | Vaccine history |
|---|---|---|---|---|---|---|---|---|
| Longo et al. (2000) [18] | France | Prospective | 117 (43 UC/74 CD) | 53/64 | 41 ± 16 | Consecutive patients with IBD | - | - |
| Biancone et al. (2001) [19] | Multicentric | Prospective | 489 IBD | - | 40 ± 13 | Consecutive patients with IBD | - | - |
| Esteve et al. (2004) [20] | Spain | Prospective | 80 CD | 45/35 | 38.2 | Prior to anti-TNF therapy | - | - |
| Tolentino et al. (2008) [21] | Brazil | Prospective | 176 (74 UC/102 CD) | 68/108 | - | Consecutive patients with IBD | 29.3/26.7 | - |
| Agmon-Levin et al. (2009) [22] | Multicentric | Prospective | 98 IBD | - | - | Patients with autoimmune disease | - | - |
| Lidar et al. (2009) [23] | Multicentric | Prospective | 119 (39 UC/80 CD) | - | - | NA | - | - |
| Loras et al. (2009) [24] | Spain | Prospective | 2,076 (928 UC/1,128 CD/20 IBD-U) | 1,043/1,033 | 44.1 ± 0.32 | Consecutive patients with IBD | - | - |
| Chevaux et al. (2010) [25] | France | Prospective | 315 (63 UC/252 CD) | - | 33.3 ± 14.1 | Consecutive patients with IBD | 33.7/ - | Yes |
| Katsanos et al. (2010) [26] | Greece | Retrospective | 453 (308 UC/145 CD) | 255/198 | 40.8 ± 11.3 | Consecutive patients with IBD | 12.1/12.1 | - |
| Morisco et al. (2012) [27] | Italy | Retrospective | 5,096 (2,611 UC/2,485 CD) | - | - | Consecutive patients with IBD | - | - |
| Park et al. (2012) [28] | Korea | Retrospective | 4,153 (1,728 UC/1,521 CD) | 2,523/1,630 | 35.1 ± 8.4 | Consecutive patients with IBD | - | - |
| Vaughn et al. (2012) [29] | USA | Retrospective | 287 (61 UC/223 CD/3 IBD-U) | 139/148 | 41.7 ± 12.3 | Only requiring anti-TNF therapy | - | - |
| Kim et al. (2013) [30] | Korea | Prospective | 513 (272 UC/241 CD) | 330/183 | 37.3 ± 13.6 | Disease duration > 6 mo | 17.3/25.5 | Yes |
| Papa et al. (2013) [31] | Italy | Prospective | 301 (117 UC/184 CD) | 142/159 | 41.9 ± 13.2 | Prior to anti-TNF therapy | 53.1/ - | Yes |
| Ben Musa et al. (2014) [32] | USA | Retrospective | 500 (206 UC/292 CD/2 IBD-U) | 195/305 | 42.5 ± 16.5 | Consecutive patients with IBD | - | - |
| Huang et al. (2014) [33] | China | Retrospective | 714 (317 UC/397 CD) | 415/299 | 37.2 ± 9.8 | Consecutive patients with IBD | 22/ - | Yes |
| Loras et al. (2014) [34] | Spain | Prospective | 389 (82 UC/307 CD) | 205/184 | 40 ± 0.7 | Prior to anti-TNF therapy | - | Yes |
| Sui et al. (2014) [35] | China | Retrospective | 256 UC | 127/119 | 50.8 ± 16.2 | Patients with UC | - | - |
| van der Have et al. (2014) [36] | Netherlands | Retrospective | 611 CD | 215/396 | 32.9 ± 13.5 | Prior to anti-TNF therapy | - | - |
| He et al. (2015) [37] | China | Retrospective | 675 (226 UC/449 CD) | 436/239 | - | Consecutive patients with IBD | - | Yes |
| Chan et al. (2016) [38] | China | Retrospective | 406 (221 UC/185 CD) | 241/165 | 44.8 ± 13.5 | IBD duration > 3 mo | 14.3/ - | - |
| Waszczuk et al. (2016) [39] | Poland | Prospective | 147 (63 UC/57 CD) | 79/68 | 38 ± 16 | Hospitalized IBD patients | - | Yes |
| Ardesia et al. (2017) [40] | Italy | Retrospective | 509 (220 UC/289 CD) | 300/209 | 42.3 ± 11.5 | Patients screened for biologic and/or thiopurine therapy | - | - |
| Chen et al. (2017) [41] | China | Retrospective | 980 (334 UC/646 CD) | 598/382 | 39.4 ± 15.5 | Hospitalized IBD patients | - | Yes |
| Harsh et al. (2017) [42] | India | Retrospective | 908 (581 UC/327 CD) | 541/367 | 37.7 ± 12.5 | Consecutive patients with IBD | - | - |
| Abid et al. (2018) [43] | Morocco | Retrospective | 755 (364 UC/391 CD) | 249/506 | 35.4 ± 11.6 | Consecutive patients with IBD | 19.7/22.2 | - |
| Shah et al. (2018) [44] | USA | Retrospective | 3,357 (775 UC/1,954 CD/628 IBD-U) | 3,013/344 | 50.6 | Only requiring anti-TNF therapy | - | - |
| Yeo et al. (2018) [45] | Korea | Prospective | 210 (109 UC/101 CD) | 133/77 | 34.9 ± 15.3 | Newly diagnosed IBD | - | Yes |
| Chou et al. (2019) [46] | Taiwan | Retrospective | 190 (110 UC/80 CD) | 135/55 | 38.4 ± 15.9 | IBD duration > 3 mo | - | - |
| Fousekis et al. (2019) [47] | Greece | Retrospective | 602 (346 UC/256 CD) | 360/242 | 39 ± 17.4 | Consecutive patients with IBD | - | - |
| Silva et al. (2019) [48] | Brazil | Prospective | 306 (165 UC/141 CD) | 117/189 | - | Consecutive patients with IBD | - | - |
| Losurdo et al. (2020) [49] | Italy | Prospective | 807 (369 UC/438 CD) | 474/333 | 46.2 ± 13.2 | Consecutive patients with IBD | - | Yes |
| Sabbah et al. (2020) [50] | Tunisia | Prospective | 74 (12 UC/62 CD) | 36/38 | 43.5 ± 14.2 | Consecutive patients with IBD | - | - |
| Patil et al. (2021) [51] | India | Prospective | 76 (42 UC/33 CD) | 48/28 | 37.5 ± 13.9 | Consecutive patients with IBD | 13.1/ - | - |
Table 2.
Summary with Sensitivity Analysis Based on Etiology, Study Design, Study Quality, and Continent of Study
REFERENCES
1. Weimers P, Munkholm P. The natural history of IBD: lessons learned. Curr Treat Options Gastroenterol 2018;16:101-111.



2. Ng SC, Tang W, Ching JY, et al. Incidence and phenotype of inflammatory bowel disease based on results from the AsiaPacific Crohn’s and Colitis Epidemiology Study. Gastroenterology 2013;145:158-165.


3. Prideaux L, Kamm MA, De Cruz PP, Chan FK, Ng SC. Inflammatory bowel disease in Asia: a systematic review. J Gastroenterol Hepatol 2012;27:1266-1280.


4. Gisbert JP, Chaparro M, Esteve M. Review article: prevention and management of hepatitis B and C infection in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2011;33:619-633.


5. Hou JK, Velayos F, Terrault N, Mahadevan U. Viral hepatitis and inflammatory bowel disease. Inflamm Bowel Dis 2010;16:925-932.


6. Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck-Ytter YT; American Gastroenterological Association Institute. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015;148:215-219.


7. Surveillance of viral hepatitis in Hong Kong-2012 update report [Internet] c2014 [cited 2022 Aug 23]. https://www.hepatitis.gov.hk/english/health_professionals/files/hepsurv12.pdf
8. Principi M, Iannone A, Losurdo G, et al. Nonalcoholic fatty liver disease in inflammatory bowel disease: prevalence and risk factors. Inflamm Bowel Dis 2018;24:1589-1596.


9. Meijer B, van Everdingen CK, Ramsoekh D, et al. Transient elastography to assess liver stiffness in patients with inflammatory bowel disease. Dig Liver Dis 2018;50:48-53.


10. Rahier JF, Magro F, Abreu C, et al. Second European evidencebased consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis 2014;8:443-468.


11. Sansone S, Guarino M, Castiglione F, et al. Hepatitis B and C virus reactivation in immunosuppressed patients with inflammatory bowel disease. World J Gastroenterol 2014;20:3516-3524.



12. Kucharzik T, Ellul P, Greuter T, et al. ECCO guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease. J Crohns Colitis 2021;15:879-913.



13. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis Of Observational Studies in Epidemiology: a proposal for reporting. JAMA 2000;283:2008-2012.


14. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71.



15. Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth 2020;18:2127-2133.

17. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-560.



18. Longo F, Hebuterne X, Tran A, et al. Prevalence of hepatitis C in patients with chronic inflammatory bowel disease in the region of nice and evaluation of risk factors. Gastroenterol Clin Biol 2000;24:77-81.

19. Biancone L, Pavia M, Del Vecchio Blanco G, et al. Hepatitis B and C virus infection in Crohn’s disease. Inflamm Bowel Dis 2001;7:287-294.


20. Esteve M, Saro C, González-Huix F, Suarez F, Forné M, Viver JM. Chronic hepatitis B reactivation following infliximab therapy in Crohn’s disease patients: need for primary prophylaxis. Gut 2004;53:1363-1365.



21. Tolentino YF, Fogaca HS, Zaltman C, Ximenes LL, Coelho HS. Hepatitis B virus prevalence and transmission risk factors in inflammatory bowel disease patients at Clementino Fraga Filho University Hospital. World J Gastroenterol 2008;14:3201-3206.



22. Agmon-Levin N, Ram M, Barzilai O, et al. Prevalence of hepatitis C serum antibody in autoimmune diseases. J Autoimmun 2009;32:261-266.


23. Lidar M, Langevitz P, Barzilai O, et al. Infectious serologies and autoantibodies in inflammatory bowel disease: insinuations at a true pathogenic role. Ann N Y Acad Sci 2009;1173:640-648.

24. Loras C, Saro C, Gonzalez-Huix F, et al. Prevalence and factors related to hepatitis B and C in inflammatory bowel disease patients in Spain: a nationwide, multicenter study. Am J Gastroenterol 2009;104:57-63.



25. Chevaux JB, Nani A, Oussalah A, et al. Prevalence of hepatitis B and C and risk factors for nonvaccination in inflammatory bowel disease patients in Northeast France. Inflamm Bowel Dis 2010;16:916-924.


26. Katsanos KH, Tsianos VE, Zois CD, et al. Inflammatory bowel disease and hepatitis B and C in Western Balkans: a referral centre study and review of the literature. J Crohns Colitis 2010;4:450-465.


27. Morisco F, Castiglione F, Rispo A, et al. Effect of immunosuppressive therapy on patients with inflammatory bowel diseases and hepatitis B or C virus infection. J Viral Hepat 2013;20:200-208.


28. Park SH, Yang SK, Lim YS, et al. Clinical courses of chronic hepatitis B virus infection and inflammatory bowel disease in patients with both diseases. Inflamm Bowel Dis 2012;18:2004-2010.


29. Vaughn BP, Doherty GA, Gautam S, Moss AC, Cheifetz AS. Screening for tuberculosis and hepatitis B prior to the initiation of anti-tumor necrosis therapy. Inflamm Bowel Dis 2012;18:1057-1063.


30. Kim ES, Cho KB, Park KS, et al. Prevalence of hepatitis-B viral markers in patients with inflammatory bowel disease in a hepatitis-B-endemic area: inadequate protective antibody levels in young patients. J Clin Gastroenterol 2014;48:553-558.

31. Papa A, Felice C, Marzo M, et al. Prevalence and natural history of hepatitis B and C infections in a large population of IBD patients treated with anti-tumor necrosis factor-α agents. J Crohns Colitis 2013;7:113-119.


32. Ben Musa R, Gampa A, Basu S, et al. Hepatitis B vaccination in patients with inflammatory bowel disease. World J Gastroenterol 2014;20:15358-15366.



33. Huang ML, Xu XT, Shen J, Qiao YQ, Dai ZH, Ran ZH. Prevalence and factors related to hepatitis B and C infection in inflammatory bowel disease patients in China: a retrospective study. J Crohns Colitis 2014;8:282-287.


34. Loras C, Gisbert JP, Saro MC, et al. Impact of surveillance of hepatitis B and hepatitis C in patients with inflammatory bowel disease under anti-TNF therapies: multicenter prospective observational study (REPENTINA 3). J Crohns Colitis 2014;8:1529-1538.


35. Sui M, Wu R, Hu X, et al. Low prevalence of hepatitis B virus infection in patients with autoimmune diseases in a Chinese patient population. J Viral Hepat 2014;21:925-929.



36. van der Have M, Belderbos TD, Fidder HH, et al. Screening prior to biological therapy in Crohn’s disease: adherence to guidelines and prevalence of infections: results from a multicentre retrospective study. Dig Liver Dis 2014;46:881-886.


37. He Y, Xu P, Chen Y, et al. Prevalence and influences of hepatitis B virus infection on inflammatory bowel disease: a retrospective study in Southern China. Int J Clin Exp Med 2015;8:8078-8085.


38. Chan HC, Wong VW, Wong GL, Tang W, Wu JC, Ng SC. Prevalence of hepatitis B and clinical outcomes in inflammatory bowel disease patients in a viral-endemic region. BMC Gastroenterol 2016;16:100.




39. Waszczuk E, Waszczuk KM, Mulak A, Paradowski L. Inadequate seroprotection against hepatitis B virus and one detected case of hepatitis C virus infection among patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol 2016;28:628-632.


40. Ardesia M, Costantino G, Mondello P, Alibrandi A, Fries W. Serology of viral infections and tuberculosis screening in an IBD population referred to a tertiary centre of Southern Italy. Gastroenterol Res Pract 2017;2017:4139656.




41. Chen D, Luo S, Ben Q, Lu L, Wan X, Wu J. Prevalence of hepatitis B and C and factors for infection and nonimmune in inflammatory bowel disease patients in China. Eur J Gastroenterol Hepatol 2017;29:509-515.


42. Harsh P, Gupta V, Kedia S, et al. Prevalence of hepatitis B, hepatitis C and human immunodeficiency viral infections in patients with inflammatory bowel disease in north India. Intest Res 2017;15:97-102.




43. Abid H, Meyiz H, Laalaj O, et al. Viral hepatitis B during chronic inflammatory bowel diseases at Fez University Hospital: prevalence and risk factors. Open J Gastroenterol 2018;8:17-26.


44. Shah R, Ho EY, Kramer JR, et al. Hepatitis B virus screening and reactivation in a national VA cohort of patients with inflammatory bowel disease treated with tumor necrosis factor antagonists. Dig Dis Sci 2018;63:1551-1557.



45. Yeo SJ, Lee HS, Jang BI, et al. Nonimmunity against hepatitis B virus infection in patients newly diagnosed with inflammatory bowel disease. Intest Res 2018;16:400-408.




46. Chou JW, Lai HC, Chang CH, Cheng KS, Feng CL, Chen TW. Epidemiology and clinical outcomes of inflammatory bowel disease: a hospital-based study in central Taiwan. Gastroenterol Res Pract 2019;2019:4175923.




47. Fousekis FS, Katsanos KH, Theopistos VI, et al. Hepatobiliary and pancreatic manifestations in inflammatory bowel diseases: a referral center study. BMC Gastroenterol 2019;19:48.




48. Silva J, Brito BS, Silva IN, et al. Frequency of hepatobiliary manifestations and concomitant liver disease in inflammatory bowel disease patients. Biomed Res Int 2019;2019:7604939.




49. Losurdo G, Iannone A, Contaldo A, et al. Chronic viral hepatitis in a cohort of inflammatory bowel disease patients from Southern Italy: a case-control study. Pathogens 2020;9:870.



50. Sabbah M, Yacoub H, Bellil N, et al. Hepatitis B and C viral infections screening in a Tunisian IBD population under immunosuppressive therapies. Rev Gastroenterol Peru 2020;40:246-251.



51. Patil AP, Simon EG, Dutta AK, Joseph AJ, Kurien RT, Chowdhury SD. Prevalence of serological markers of hepatitis B in inflammatory bowel disease: experience from a tertiary care centre in South India. Trop Doct 2021;51:326-331.



52. Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 2018;3:383-403.

53. GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020;5:17-30.

54. Childs L, Roesel S, Tohme RA. Status and progress of hepatitis B control through vaccination in the South-East Asia region, 1992-2015. Vaccine 2018;36:6-14.



55. Loras C, Gisbert JP, Mínguez M, et al. Liver dysfunction related to hepatitis B and C in patients with inflammatory bowel disease treated with immunosuppressive therapy. Gut 2010;59:1340-1346.


56. Lee JM, Wei SC, Lee KM, et al. Clinical course of hepatitis B viral infection in patients undergoing anti-tumor necrosis factor α therapy for inflammatory bowel disease. Gut Liver 2022;16:396-403.



57. Lamb CA, Kennedy NA, Raine T, et al. British society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019;68(Suppl 3):s1-s106.



58. Kochhar GS, Mohan BP, Khan SR, et al. Hepatitis-B vaccine response in inflammatory bowel disease patients: a systematic review and meta-analysis. Inflamm Bowel Dis 2021;27:1610-1619.



59. Gupta A, Macrae FA, Gibson PR. Vaccination and screening for infections in patients with inflammatory bowel disease: a survey of Australian gastroenterologists. Intern Med J 2011;41:462-467.


60. Poupardin C, Nahon S, Pariente A, Cadranel JF, Renou C; ANGH. Hepatitis B reactivation in patients with inflammatory bowel disease: a prospective survey on screening and prevention practices at general hospitals in France. Inflamm Bowel Dis 2011;17:669-670.

61. Polaris Observatory HCV Collaborators. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol 2022;7:396-415.

62. Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatitis C virus infection: an up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol 2016;22:7824-7840.



63. European Centre for Disease Prevention and Control. Prevention of hepatitis B and C in the EU/EEA and the UK [Internet]. c2020 [cited 2022 Aug 23]. https://www.ecdc.europa.eu/sites/default/files/documents/hepatitis-B-and-C-prevention_1.pdf
- TOOLS
-
 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link XML Download
XML Download-
 Full text via DOI
Full text via DOI
-
 Download Citation
Download Citation
 Supplement1
Supplement1 Supplement2
Supplement2 Supplement3
Supplement3 Supplement4
Supplement4 Supplement5
Supplement5 Supplement6
Supplement6 Supplement7
Supplement7 Supplement8
Supplement8 Supplement9
Supplement9 Supplement10
Supplement10 Supplement11
Supplement11 Supplement12
Supplement12 Supplement13
Supplement13 Supplement14
Supplement14 Supplement15
Supplement15 Supplement16
Supplement16 Supplement17
Supplement17 Supplement18
Supplement18 Supplement19
Supplement19








