 |
 |
- Search
| Intest Res > Volume 20(4); 2022 > Article |
|
Abstract
Background/Aims
Methods
Results
ADDITIONAL INFORMATION
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
Puri AS is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author Contribution
Conceptualization: Puri AS, Mishra A. Data curation: Mishra A. Formal analysis: Puri AS, Sachdeva S, Dalal A. Investigation: Puri AS, Mishra A. Methodology: Puri AS, Mishra A. Project administration: Mishra A. Supervision: Puri AS. Validation: Sachdeva S, Dalal A. Visualization: Sachdeva S, Dalal A. Writing -original draft: Puri AS, Mishra A. Writing - review & editing: Puri AS, Sachdeva S, Dalal A. Approval of final manuscript: All authors.
Fig. 1.
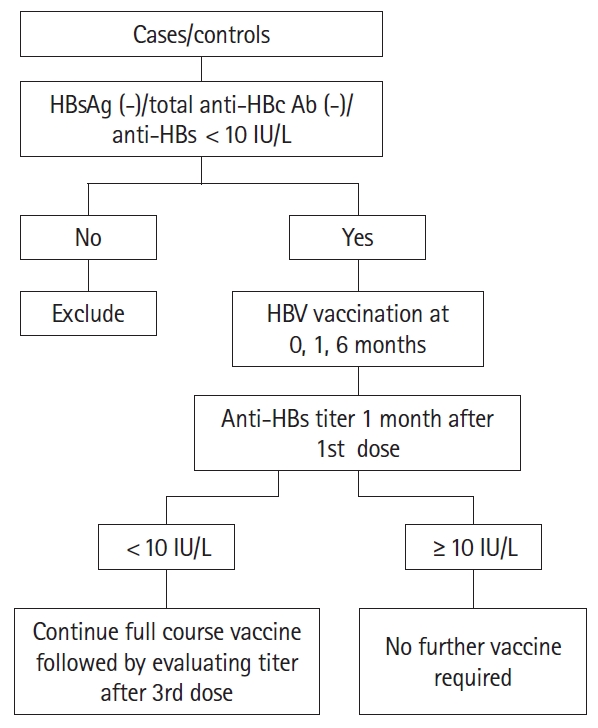
Table 1.
Table 2.
Table 3.
Table 4.
| Author (year) | Country | Study type |
No. of IBD patients |
HBV vaccine response (%) | |
|---|---|---|---|---|---|
| UC | CD | ||||
| Vida Pérez et al. (2009) [14] | Spain | Retrospective | 45 | 55 | 34 |
| Altunöz et al. (2012) [1] | Turkey | Prospective | 62 | 38 | 76 |
| Gisbert et al. (2012) [15] | Spain | Prospective | 31 | 69 | 60 |
| Sempere et al. (2013) [16] | Spain | Retrospective | 24 | 76 | 48 |
| Andrade et al. (2015) [17] | Portugal | Prospective | 21 | 79 | 76 |
| Belle et al. (2015) [18] | France | Retrospective | 20 | 80 | 79 |
| Cekic et al. (2015) [19] | Turkey | Retrospective | 52 | 48 | 57 |
| Cossio-Gil et al. (2015) [20] | Spain | Retrospective | 59 | 110 | 51 |
| Etzion et al. (2016) [21] | Israel | RCT | 21 | 79 | 75 |
| Jiang et al. (2017) [13] | China | Meta-analysis | 426 | 732 | 61 |
| Chang et al. (2018) [12] | Korea | Prospective | 28 | 45 | 89 |
| Pratt et al. (2018) [22] | USA | Retrospective | 137 | 246 | 55 |
| Current study (2020) | India | Prospective | 100 | 0 | 82 |
REFERENCES
- TOOLS








