 |
 |
- Search
| Intest Res > Epub ahead of print |
|
Abstract
Background/Aims
Methods
Results
ADDITIONAL INFORMATION
Conflict of Interest
Matsuoka K has received lecture fees from Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., AbbVie LLC, EA Pharma Co., Ltd., and Pfizer Japan Inc., Mochida Pharmaceutical Co., Ltd.; research funding from Janssen Pharmaceutical K.K.; scholarship funding from AbbVie LLC, EA Pharma Co., Ltd., Mochida Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., and Mitsubishi Tanabe Pharma Corporation. Inoue T, Tsuchiya H, Nagano K, and Iwahori T are employees of Janssen Pharmaceutical K.K. Iwahori T received lecture fees from Shiga University of Medical Science and YahadClub for Doctors Physicians in Israel. Matsuoka K is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author Contributions
Conceptualization: Matsuoka K, Inoue T, Nagano K. Data curation: Tsuchiya H. Formal analysis: Tsuchiya H. Funding acquisition: Inoue T, Nagano K. Investigation: Inoue T, Tsuchiya H, Nagano K, Iwahori T. Methodology: Tsuchiya H, Iwahori T. Project administration: Inoue T. Supervision: Matsuoka K. Validation: Tsuchiya H. Visualization: Tsuchiya H. Writing - original draft: Inoue T. Writing - review & editing: Matsuoka K, Tsuchiya H, Nagano K, Iwahori T. Approval of final manuscript: all authors.
Additional Contributions
Miotsukushi Analytics Co., Ltd were commissioned to perform the analysis work for this research, and Yu Kimura and Keita Wakimoto acted as persons in charge. The authors also thank Yoshiko Okamoto, PhD, and Mark Snape, MBBS, of in Science Communications, Springer Healthcare, for helping write the outline and first draft of the manuscript.
Supplementary Material
Supplementary Table 2.
Supplementary Table 3.
Supplementary Table 4.
Supplementary Table 5.
Fig.┬Ā1.
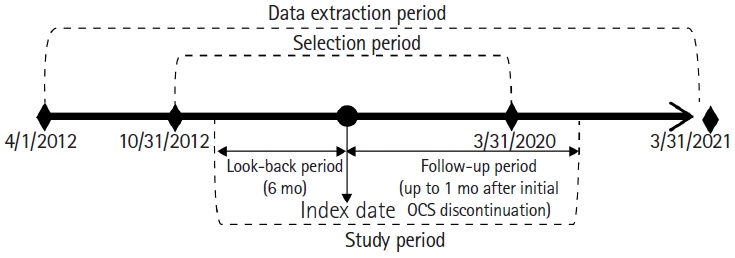
Fig.┬Ā2.
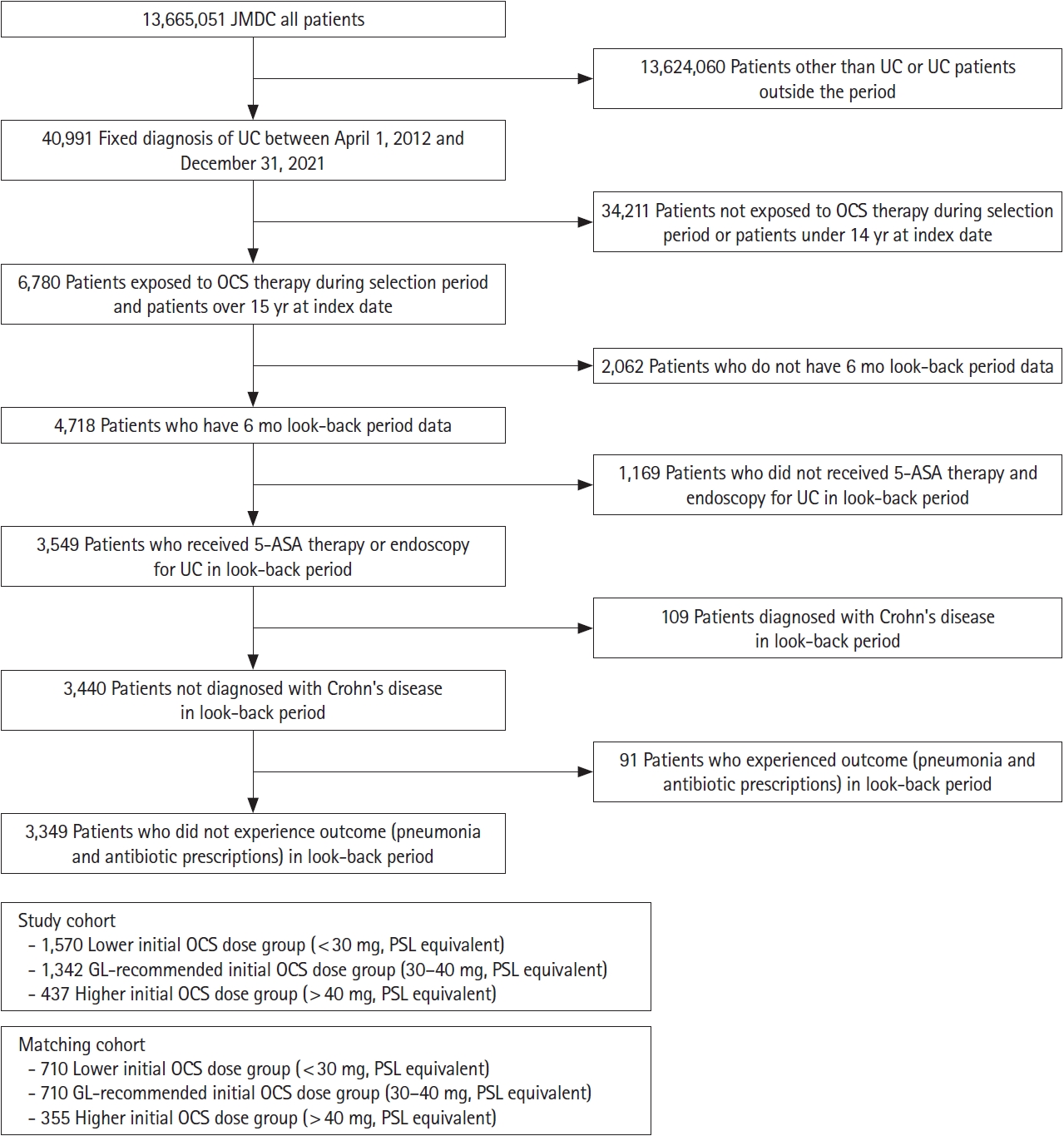
Fig.┬Ā3.
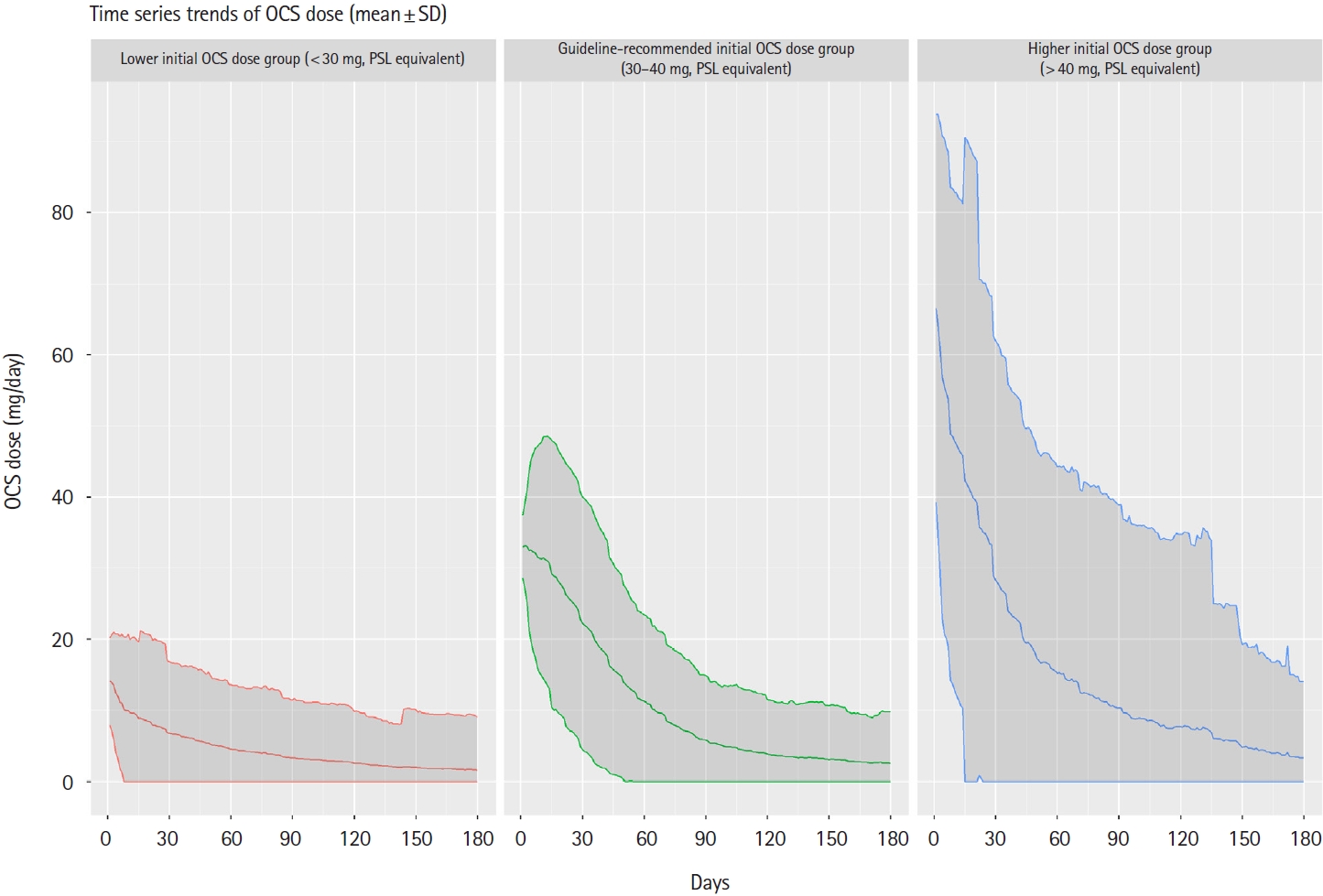
Fig.┬Ā4.

Fig.┬Ā5.
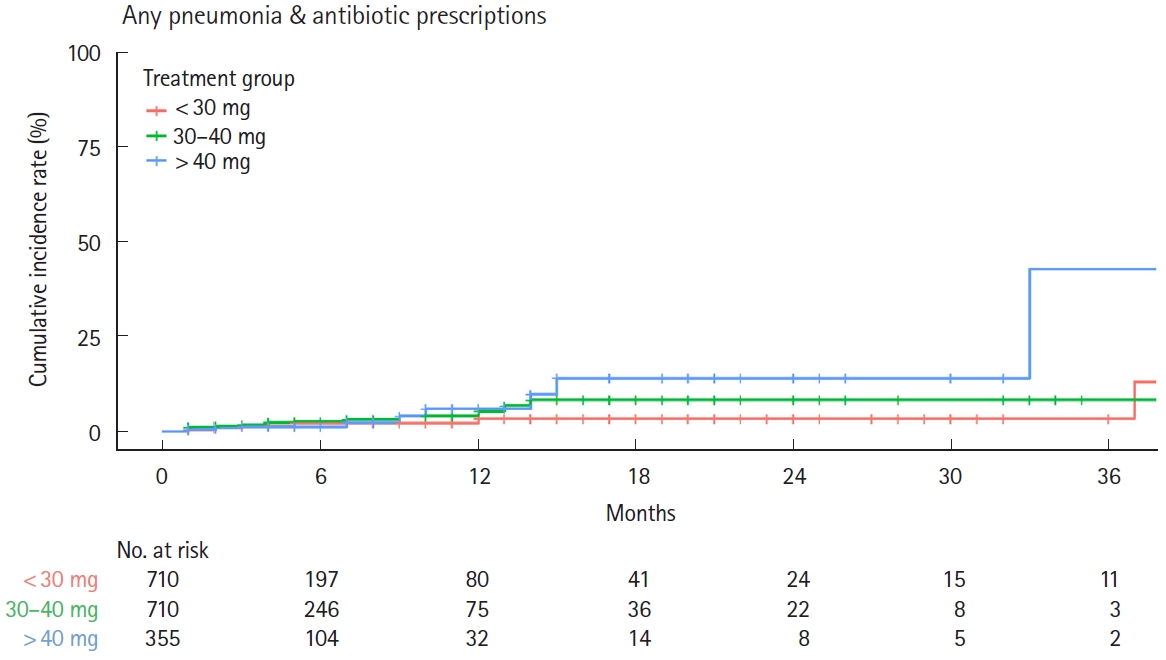
Fig.┬Ā6.
Table┬Ā1.
| Baseline characteristics |
Before PS matching |
After PS matching |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <30 mg (n=1,570) | 30-40 mg (n=1,342) | >40 mg (n=437) | SMDa | SMDb | < 30 mg (n = 710) | 30-40 mg (n = 710) | > 40 mg (n = 355) | SMDa | SMDb | ||
| Age (yr) | Mean ┬▒ SD | 42.7 ┬▒ 12.3 | 39.5 ┬▒ 13.7 | 41.8 ┬▒ 12.2 | 0.246 | 0.178 | 41.3 ┬▒ 12.62 | 41.3 ┬▒ 13.43 | 41.6 ┬▒ 12.19 | 0.002 | 0.021 |
| Median (IQR) | 44 (34-52) | 40 (28-50) | 42 (34-50) | 42 (32-51) | 42 (31-51) | 42 (33-50) | |||||
| Age category 1 | Ōēż 19 yr | 61 (3.88) | 108 (8.05) | 20 (4.58) | 0.297 | 0.289 | 44 (6.20) | 48 (6.76) | 18 (5.07) | 0.107 | 0.152 |
| 20-29 yr | 200 (12.73) | 272 (20.27) | 58 (13.27) | 101 (14.23) | 112 (15.77) | 47 (13.24) | |||||
| 30-39 yr | 347 (22.10) | 279 (20.78) | 95 (21.74) | 158 (22.25) | 146 (20.56) | 82 (23.10) | |||||
| 40-49 yr | 459 (29.23) | 318 (23.70) | 144 (33.00) | 205 (28.87) | 194 (27.3) | 111 (31.27) | |||||
| 50-59 yr | 382 (24.33) | 266 (19.82) | 86 (19.68) | 153 (21.55) | 151 (21.32) | 73 (20.56) | |||||
| 60-69 yr | 112 (7.13) | 88 (6.56) | 32 (7.32) | 46 (6.48) | 51 (7.18) | 22 (6.20) | |||||
| Ōēź 70 yr | 9 (0.57) | 11 (0.82) | 2 (0.48) | 3 (0.42) | 8 (1.13) | 2 (0.56) | |||||
| Age category 2 (elderly) | 15-64 yr | 1,532 (97.58) | 1,309 (97.54) | 426 (97.48) | 0.003 | 0.004 | 699 (98.45) | 689 (97.04) | 347 (97.75) | 0.095 | 0.044 |
| Ōēź 65 yr | 38 (2.42) | 33 (2.46) | 11 (2.52) | 11 (1.55) | 21 (2.96) | 8 (2.25) | |||||
| Sex | Male | 902 (57.45) | 861 (64.16) | 281 (64.30) | 0.138 | 0.003 | 443 (62.39) | 452 (63.66) | 220 (61.97) | 0.026 | 0.035 |
| Female | 668 (42.55) | 481 (35.84) | 156 (35.70) | 267 (37.61) | 258 (36.34) | 135 (38.03) | |||||
| Smoking history | No | 793 (50.51) | 590 (43.96) | 212 (48.51) | 0.087 | 0.029 | 364 (51.27) | 346 (48.73) | 179 (50.42) | 0.073 | 0.028 |
| Yes | 137 (8.73) | 129 (9.61) | 50 (11.44) | 63 (8.87) | 73 (10.28) | 35 (9.86) | |||||
| Unknown | 640 (40.76) | 623 (46.42) | 175 (40.05) | 283 (39.86) | 291 (40.99) | 141 (39.72) | |||||
| BMI (kg/m2) | Mean ┬▒ SD | 22.6 ┬▒ 3.24 | 22.9 ┬▒ 3.76 | 23.0 ┬▒ 3.55 | 0.083 | 0.029 | 22.6 ┬▒ 3.19 | 22.7 ┬▒ 3.33 | 22.8 ┬▒ 3.33 | 0.041 | 0.032 |
| Median (IQR) | 22.1 (20.3-24.5) | 22.5 (20.3-24.9) | 22.7 (20.5-25) | 22.2 (20.3-24.4) | 22.4 (20.2-24.6) | 22.6 (20.5-24.9) | |||||
| BMI categoryc | Underweight | 68 (4.33) | 59 (4.40) | 17 (3.89) | 0.100 | 0.073 | 29 (4.08) | 31 (4.37) | 15 (4.23) | 0.038 | 0.042 |
| Normal weight | 702 (44.71) | 515 (38.38) | 196 (44.85) | 311 (43.80) | 326 (45.92) | 156 (43.94) | |||||
| Obesity 1 | 181 (11.53) | 154 (11.48) | 60 (13.72) | 86 (12.11) | 83 (11.69) | 46 (13.00) | |||||
| Obesity 2 | 30 (1.91) | 34 (2.53) | 12 (2.75) | 13 (1.83) | 12 (1.69) | 8 (2.25) | |||||
| Unknown | 589 (37.52) | 580 (43.22) | 152 (34.78) | 271 (38.17) | 258 (36.33) | 130 (36.62) | |||||
| CCI | Mean (SD) | 0.74 ┬▒ 1.23 | 0.60 ┬▒ 1.06 | 0.84 ┬▒ 1.46 | 0.119 | 0.189 | 0.62 ┬▒ 0.98 | 0.63 ┬▒ 1.08 | 0.72 ┬▒ 1.22 | 0.005 | 0.079 |
| Median (IQR) | 0 (0-1) | 0 (0-1) | 0 (0-1) | 0 (0-1) | 0 (0-1) | 0 (0-1) | |||||
| CCI category | Low (0) | 884 (56.31) | 829 (61.77) | 250 (57.21) | 0.126 | 0.156 | 413 (58.17) | 427 (60.14) | 212 (59.72) | 0.079 | 0.066 |
| Medium (1-2) | 581 (37.01) | 451 (33.61) | 152 (34.78) | 269 (37.89) | 247 (34.79) | 121 (34.08) | |||||
| High (3-4) | 82 (5.22) | 48 (3.58) | 24 (5.49) | 23 (3.24) | 29 (4.08) | 16 (4.51) | |||||
| Very high (Ōēź 5) | 23 (1.46) | 14 (1.04) | 11 (2.52) | 5 (0.70) | 7 (0.99) | 6 (1.69) | |||||
| Comorbidity | Allergic disease | 635 (40.45) | 434 (32.34) | 146 (33.41) | 0.169 | 0.023 | 258 (36.34) | 246 (34.65) | 125 (35.21) | 0.035 | 0.012 |
| Rheumatic disease | 30 (1.91) | 9 (0.67) | 5 (1.14) | 0.110 | 0.050 | 6 (0.85) | 7 (0.99) | 5 (1.41) | 0.015 | 0.039 | |
| Concomitant drug | 5-ASA | 1,468 (93.50) | 1,284 (95.68) | 408 (93.36) | 0.096 | 0.102 | 681 (95.92) | 678 (95.49) | 339 (95.49) | 0.021 | 0.000 |
| AZA | 81 (5.16) | 75 (5.59) | 27 (6.18) | 0.019 | 0.025 | 39 (5.49) | 41 (5.77) | 22 (6.20) | 0.012 | 0.018 | |
| 6-MP | 13 (0.83) | 12 (0.89) | 7 (1.60) | 0.007 | 0.064 | 3 (0.42) | 4 (0.56) | 2 (0.56) | 0.020 | 0.000 | |
| Tacrolimus | 22 (1.40) | 4 (0.30) | 1 (0.23) | 0.120 | 0.013 | 0 | 0 | 0 | 0.000 | 0.000 | |
| Biologics | 72 (4.59) | 38 (2.83) | 20 (4.58) | 0.093 | 0.092 | 25 (3.52) | 22 (3.10) | 7 (1.97) | 0.024 | 0.072 | |
| JAK-inhibitor | 1 (0.06) | 2 (0.15) | 0 | 0.026 | 0.055 | 0 | 0 | 0 | 0.000 | 0.000 | |
| No. of beds at a medical facility | 0-19 | 924 (58.85) | 325 (24.22) | 142 (32.49) | 0.760 | 0.225 | 262 (36.90) | 264 (37.18) | 132 (37.18) | 0.031 | 0.092 |
| 20-99 | 83 (5.29) | 107 (7.97) | 25 (5.72) | 50 (7.04) | 51 (5.77) | 22 (6.20) | |||||
| 100-199 | 73 (4.65) | 105 (7.82) | 29 (6.64) | 53 (7.46) | 48 (6.76) | 26 (7.32) | |||||
| 200-299 | 58 (3.69) | 71 (5.29) | 16 (3.66) | 36 (5.07) | 35 (4.93) | 13 (3.66) | |||||
| 300-499 | 156 (9.94) | 303 (22.58) | 79 (18.08) | 118 (16.62) | 122 (17.18) | 58 (16.34) | |||||
| Ōēź 500 | 276 (17.58) | 431 (32.12) | 146 (33.41) | 191 (26.90) | 190 (26.76) | 104 (29.30) | |||||
| Prophylactic TMP-SMX | No | 1,518 (96.69) | 1,119 (83.38) | 385 (88.10) | 0.455 | 0.135 | 695 (97.89) | 693 (97.61) | 346 (97.46) | 0.019 | 0.009 |
| Yes | 52 (3.31) | 223 (16.62) | 52 (11.90) | 15 (2.11) | 17 (2.39) | 9 (2.54) | |||||
| Hospitalization at index date | No | 1,307 (83.25) | 845 (62.97) | 343 (78.49) | 0.470 | 0.346 | 574 (80.85) | 581 (81.83) | 289 (81.41) | 0.025 | 0.011 |
| Yes | 263 (16.75) | 497 (37.03) | 94 (21.51) | 136 (19.15) | 129 (18.17) | 66 (18.59) | |||||
c Underweight (<18.5 kg/m2), normal weight (Ōēź18.5 and <25.0 kg/m2), obesity 1 (Ōēź25.0 and <30.0 kg/m2), obesity 2 (Ōēź30.0 kg/m2).
PS, propensity score; SMD, standardized mean difference; SD, standard deviation; IQR, interqurtile range; BMI, body mass index; CCI, Charlson Comorbidity Index; 5-ASA, 5-aminosalicylic acid; AZA, azathioprine; 6-MP, 6-mercaptopurine; JAK, Janus kinase inhibitor; TMP-SMX, trimethoprim/sulfamethoxazole.
Table┬Ā2.
Table┬Ā3.
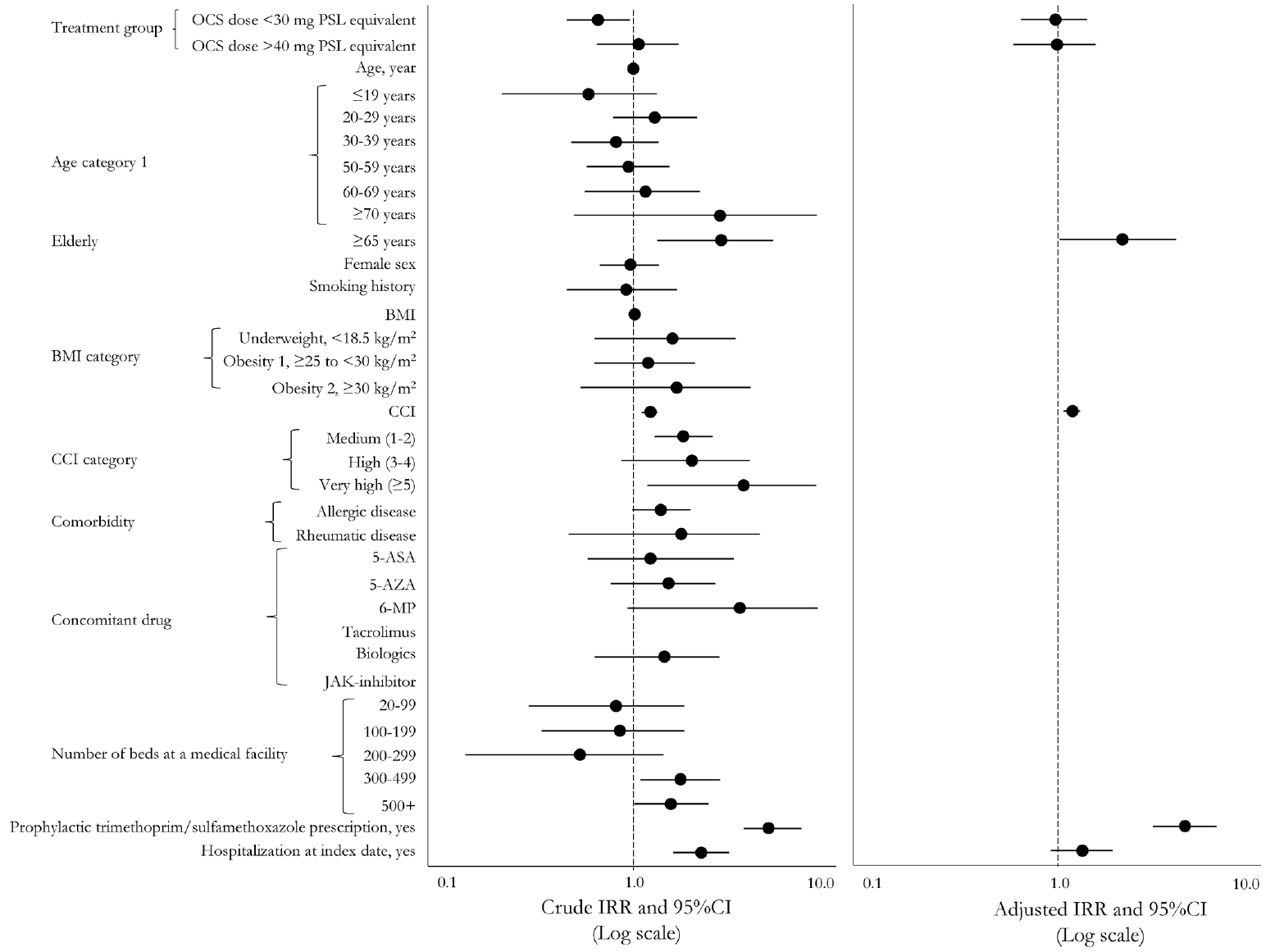
PY, patient year; IR, incidence rate; IRR, incidence rate ratio; CI, confidence interval; OCS, oral corticosteroids; GL, guideline; PSL, prednisolone; BMI, body mass index; CCI, Charlson Comorbidity Index; 5-ASA, 5-aminosalicylic acid; AZA, azathioprine; 6-MP, 6-mercaptopurine; JAK, Janus kinase inhibitor; TMP-SMX, trimethoprim/sulfamethoxazole.








