|
 |
- Search
| Intest Res > Volume 13(1); 2015 > Article |
|
Abstract
Background/Aims
Ghrelin levels are known to increase in patients with ulcerative colitis (UC), but serum obestatin levels in UC patients are not well elucidated. The aim of this study was to examine the relationship between serum ghrelin and obestatin levels and disease activity in UC patients.
Methods
The serum ghrelin and obestatin levels were measured in 21 UC patients (12 with active disease and 9 in remission) using enzyme-linked immunosorbent assay. The relationship between the circulating levels of these 2 hormones and disease activity was analyzed. The colonic mucosal mRNA expression of ghrelin and obestatin was measured by quantitative reverse transcription polymerase chain reaction.
Results
The mean serum ghrelin values were significantly higher in patients with active disease than in patients with remission (1370.6±404.3 vs. 783.5±235.3 pg/mL, P=0.001). Colonic mucosal mRNA expression of ghrelin was also significantly higher in patients with active disease than in patients in remission (0.805±0.214 vs. 0.481±0.356, P=0.018). However, the mean serum obestatin levels and colonic mucosal mRNA expression of obestatin were not significantly different between both groups. The circulating obestatin/ghrelin ratio was significantly lower in patients with active UC than in patients in remission (0.32±0.08 vs. 0.58±0.20, P=0.001).
Ulcerative colitis (UC) is an immune-mediated process that denudes the intestinal mucosa and can cause reduced appetite, weight loss, and systemic inflammation.1 The pathogenesis of UC is dependent on cellular immune reactions and inflammatory cytokines in response to different genetic, immune and environmental factors.2 However, the molecular mechanisms by which this occurs are not yet fully defined. Recently, it was reported that the dysregulation of cytokine secretion by adipose tissue, including adiponectin, resistin, leptin, and ghrelin, played a significant role in the pathogenesis of IBD.3
Ghrelin is the only O-octanoylated peptide and plays a role in C8 fatty acid attachment to a serine moiety by the enzyme.4 Ghrelin has receptors that can directly stimulate growth hormone secretion in the pituitary and on energyregulating centers in the hypothalamus to promote adipogenesis in the adipose tissue.5,6,7 Furthermore, ghrelin acts on lymphocyte receptors to provide anti-inflammatory effects, including a decrease in circulating cytokines.8,9 Some reports demonstrated a strong relationship between the serum ghrelin level and the severity of mucosal inflammation in the gastrointestinal tract.10,11
A recent study demonstrated high serum ghrelin levels in patients with IBD, including CD and UC.12 Another study also showed a close relationship between ghrelin levels and the severity of intestinal inflammation in patients with celiac disease.13 In contrast to ghrelin, obestatin acts as an anorexic hormone by reducing food intake, gastric emptying time, jejunal motility, and body-weight gain.14 It has been suggested that the obestatin/ghrelin ratio could be used as a surrogate marker of IBD activity.15 There have been few studies to measure the serum ghrelin levels by ELISA and colonic mucosal ghrelin mRNA expression by quantitative RT-PCR (qRT-PCR) in the same patients. In addition, no studies have yet investigated the relationship between serum obestatin levels and UC disease activity in Korean patients.
The aim of this study was to define the relationship between ghrelin, obestatin, the obestatin/ghrelin ratio and disease activity in patients with UC.
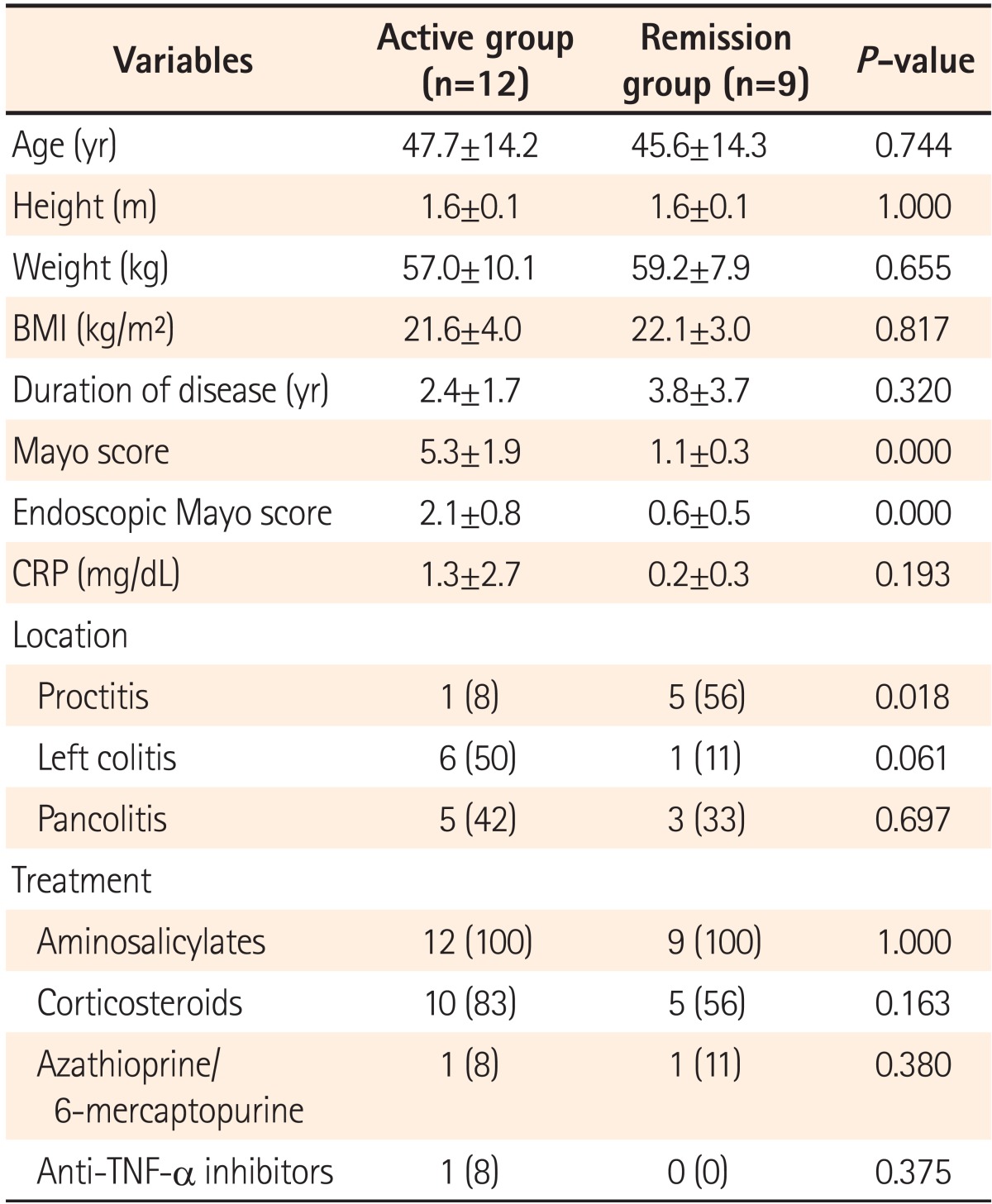
Twenty-one consecutive patients with UC (treated and followed at the Seoul National University Boramae Hospital) were enrolled in this study from July 2011 to June 2012. The diagnosis of UC was based on clinical, endoscopic, and histopathological findings.16 The disease was classified as either active or in remission, and the disease activity in UC patients was assessed with the Mayo score index as described by Seo et al.17 Clinical remission was defined as a total Mayo score of 2 or less with every sub-score less than 2. Disease activity of UC was evaluated at the time of serum collection according to the total Mayo score. The clinical demographic characteristics and therapeutic management strategies are shown in Table 1.
Serum laboratory measurements included red and white blood cell count, hemoglobin, hematocrit, platelet count, total protein, albumin, cholesterol, triglycerides, ESR, and CRP and were routinely obtained from all patients when a colonoscopy was performed. CRP was measured as a marker of inflammation. The cut-off point of normal versus increased CRP levels was determined to be 0.49 mg/dL. Patients who had any chronic systemic disease, such as hypo- or hyperthyroidism, adrenal insufficiency, diabetes mellitus, hypercholesterolemia, hypertriglyceridemia, malignant hypertension, coronary heart disease, chronic obstructive pulmonary disease, or any other malignancy or autoimmune disease, were excluded from this study. The study protocol was approved by the Institutional Review Board at Seoul National University Boramae Hospital (IRB No. 06-2010-170).
Blood samples were obtained in the morning after overnight fasting. After centrifugation (3,800 g for 15 minutes), 1-2 mL of serum was stored at -80℃ until assayed. Obestatin concentrations were measured with the commercially available YK231 Human Obestatin EIA kit (Yanaihara Institute, Shizuoka, Japan; detection range: 0.231-258 ng/mL). Ghrelin concentrations were measured using commercially available Ghrelin EIA kits (Phoenix Pharmaceuticals, Brulingame, CA, USA; detection range: 0-100 ng/mL). Standards in the commercial kit were diluted according to the manufacturer's instructions, and serum samples were added to wells coated with ghrelin- and obestatin-specific antibodies. Stop solution was added to each well, and the absorbance values were determined by a spectrophotometric ELISA-Reader (Microplate Reader, Biotek Instruments Inc., Winooski, VT, USA).
Pathological mucosal lesions in the colonic mucosa were biopsied during colonoscopic examinations. The mRNA expression of ghrelin and obestatin was quantified using qRT-PCR. Total RNA was extracted and purified from colitis mucosal biopsy specimens using Trizol (GIBCO, Gaitherburg, MD, USA). qRT-PCR analysis was performed using the Light-Cycler 480 DNA SYBR Green I Master and a LightCycler 480 system (Roche Applied Science, Mannheim, Germany) with annealing at 60℃. The nucleotide sequences of the primers were as follows. Obestatin forward sense: AAG ATG GAG GTC AAG CAG AAG; reverse sense GAC AGC TTG ATT CCA ACA TCA; ghrelin forward sense: CAG CTT CCT GAG CCC TGA A; reverse sense CTT GGC TGG TGG CTT CTT C.
The data are presented as means±SDs. The Mann-Whitney U test and Kruskal-Wallis test were used to compare pairs of independent continuous variables, and the Fisher's exact test was used to compare categorical variables between the two groups of UC (active disease vs. remission). Correlation between the serum ghrelin and CRP levels was determined with Spearman's correlation analysis, and a level of P<.0.05 was considered significant. The statistical analysis was performed with SPSS (SPSS version 20.0; IBM, New York, USA) for Windows.
A total of 21 UC patients were included in the study. Of the patients with UC, 9 (42%) were in remission, and 12 (58%) had active disease. The 12 patients with active UC included 6 males and 6 females with a median age of 52 years. The 9 patients in remission included 7 males and 2 females with a median age of 45 years. The median durations of active UC and inactive UC were 2.37 years and 3.75 years. Of the patients with active UC, 8.3% (n=1) had proctitis; 50% (n=6) had left-sided colitis; and 41.6% (n=5) had pancolitis. There was no significant difference between the active and inactive patients with respect to age, gender, BMI, or duration of disease (P>.0.05) (Table 1).
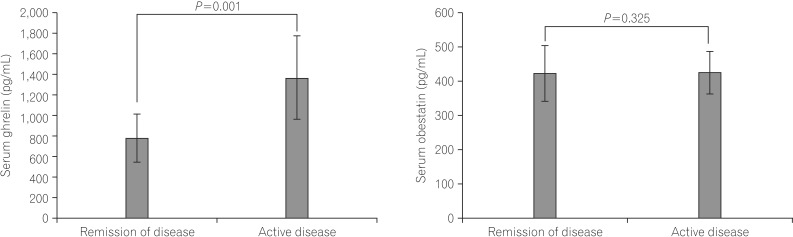
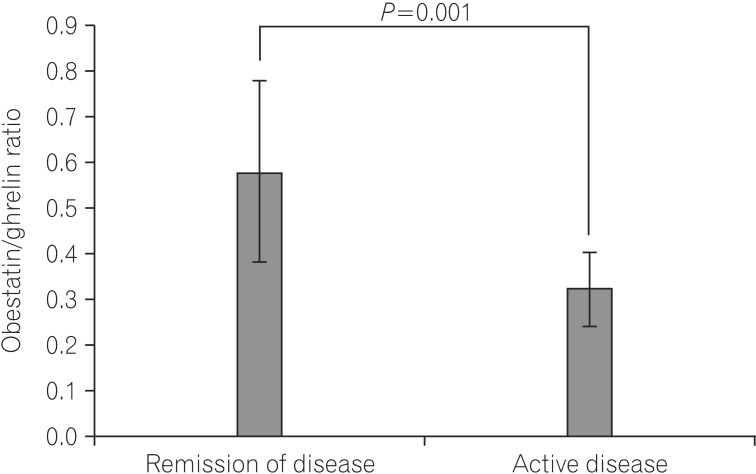
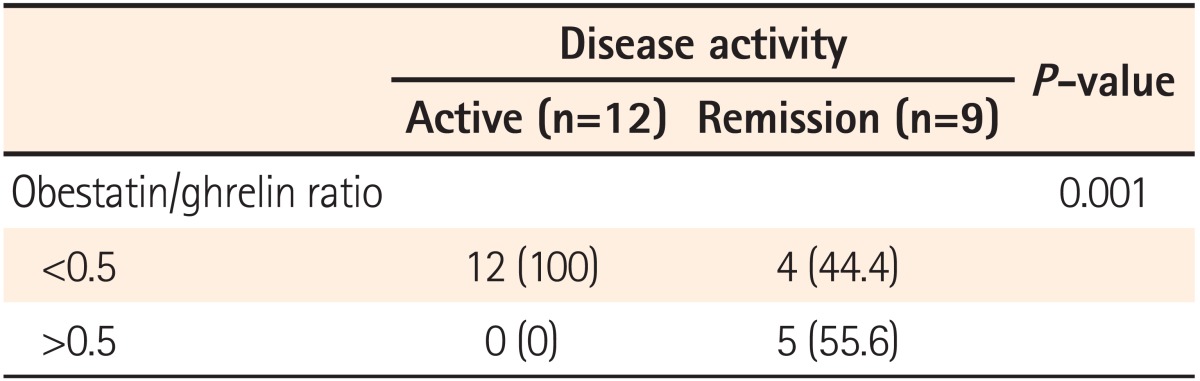
The mean serum ghrelin levels were significantly higher in patients with active UC (1370.6±404.3 pg/mL) compared to patients in remission (783.5±235.3 pg/mL, P=0.001). However, the mean serum obestatin levels were not significantly different between UC patients with active disease and patients in remission (424.1±81.0 vs. 421.2±61.5 pg/mL, P=0.325) (Fig. 1). The ratio of serum obestatin/ghrelin concentration was statistically lower in patients with active UC (0.32±0.08) compared to patients in remission (0.58±0.20, P=0.001) (Fig. 2).
Patients with ratio values less than 0.5 have a greater likelihood of suffering from active disease than patients with ratio values greater than 1 (100 vs. 0%) (Table 2).
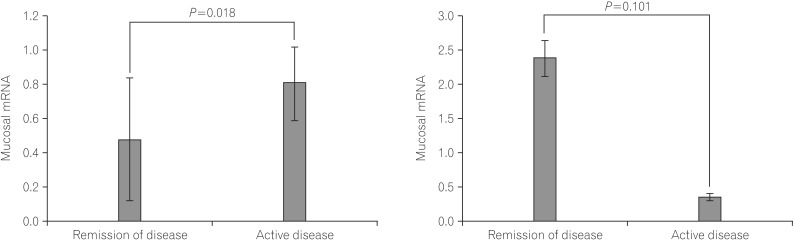
The colonic mucosal mRNA expression of ghrelin was significantly higher in patients with active UC (0.805±0.214) compared to patients in remission (0.481±0.356, P=0.018). The colonic mucosal mRNA expression of obestatin demonstrated no significant differences between patients with active UC (0.370±0.047) and patients in remission (2.390±0.261, P=0.101) (Fig. 3). The ratio of colonic mucosal obestatin/ghrelin mRNA expression was not significantly different between the groups.
There was no correlation between serum ghrelin and obestatin levels and disease location in either group. Only serum ghrelin levels were significantly higher in patients with pancolitis (1088.0±89.0 pg/mL) compared to left-sided colitis (604.3±98.0 pg/mL) among active UC patients (P=0.011). Mean serum ghrelin levels were positively correlated with CRP levels (Spearman's coefficient=0.618, P=0.003), and the serum ghrelin/obestatin ratio demonstrated a negative correlation with CRP levels (Spearman's coefficient=-0.628, P=0.002).
In the present study, we measured serum ghrelin and obestatin levels and their ratio in patients with UC according to disease activity and demonstrated that serum ghrelin level was increased and the serum obestatin/ghrelin ratio was decreased in patients with active UC. However, there was no relationship between the serum obestatin level and disease activity in patients with UC.
Previously, changes in serum values of adipokines after treatment, including leptin, adiponectin, resistin and visfatin, were reported in Korean patients with IBD according to disease activity.18 In this study, although the serum visfatin levels were reduced significantly after the induction of therapy in patients with active IBD, the serum levels of leptin, adiponectin, and resistin did not change significantly with therapy. The relationship between ghrelin, obestatin and IBD disease activity has not reported in Korean patients with IBD, and the present study may be the first report concerning Korean patients with UC.
It has been reported that serum ghrelin levels were significantly increased in patients with IBD compared to healthy controls.3 A previous study by Homosi et al. demonstrated that ghrelin mRNA levels in the colonic mucosa of patients with IBD were higher than those of controls, and growth hormone secretagogue receptor type 1a, a specific receptor for ghrelin, was also significantly higher in patients with IBD than in controls.19 Alexandridis et al. found that the ratio of obestatin/ghrelin was closely related to disease activity in patient with IBD, and patients with active disease had a ratio less than 1.15 In the present study, serum ghrelin values in patients with active UC were increased, whereas serum obestatin values were not different according to disease activity. The serum obestatin/ghrelin ratio in patients with active UC was significantly lower compared to that in patients in remission, and this result was consistent with the previous study although patients with active UC had a ratio less than 0.5 in the present study.
The exact mechanism of serum ghrelin level increase in active IBD patients is not fully understood, but increased mesenteric white adipose tissue (WAT) in IBD patients, especially CD, plays an important role in this phenomenon. Mesenteric WAT is known to a source of adipokines and stimulate intestinal inflammation by crosstalk between adipose tissue and intestinal mucosa.20 It has been reported that adipokines originated from creeping fat, namely progressive expansion of the mesenteric WAT, play a critical role in development of intestinal inflammation and elevated ghrelin in patients with CD.21,22 However, creeping fat is rare in patients with active UC, and a relationship between ghrelin and creeping fat is not clear in patients with active UC (unlike CD).
The clinical significance of increased ghrelin levels was not well understood in patients with UC. Administration of ghrelin in most animal models of colitis resulted in improved disease activity and systemic inflammation.23,24 A previous study found increased levels of T helper-2 cytokines (interleukin-4 and interleukin-13) and inhibition of interferon-γ production after T cell stimulation with ghrelin, indicating that ghrelin directly polarizes T cells toward T helper-2 responses.19 The mechanism for these effects is not fully understood but might be related to decrease of mucosal inflammation and increase of intestinal motility, appetite, and colonic blood flow. In the present study, increased ghrelin mRNA expression in the colonic mucosa of patients with active UC indicated that ghrelin might be involved in the pathogenesis of UC by regulating the immune response at the mucosal level.
We compared serum ghrelin levels according to the location of disease extent in patients with UC. There was no significant association between serum ghrelin levels and disease location in patients with UC. Although serum ghrelin levels were different between proctitis and pancolitis, the ghrelin levels were not significantly different among all 3 subgroups according to disease location in patients with active UC, by post-hoc analysis of Kruskal-Wallis test, which may be due to small sample size in each subgroup. These findings are similar to those of previous studies in which serum adipocytokine levels and disease location were not associated in UC or CD patients.3 In our study, a correlation was found between serum ghrelin levels and CRP in patients with UC (P=0.003). Our results suggest that serum ghrelin levels in patients with UC may be used as a disease activity marker similar to CRP. Yamamoto et al. reported an inverse correlation between adiponectin concentrations and serum CRP levels in patients with CD.25
This study has several limitations. First, the patients in this study were enrolled retrospectively. Therefore, the measurements of ghrelin and obestatin levels were not performed at the initial diagnosis of UC. Additionally, a healthy control group could not be enrolled and compared to patients with active UC. Second, we did not measure the ghrelin levels before and after treatment of UC. Third, a small number of patients were enrolled. Although we could not show the relationship between ghrelin levels and subgroups in active UC patients because of the small sample size, we identified a trend of increased ghrelin level according to disease severity of UC. Fourth, in this study, other adipocytokines (such as leptin and adiponectin) was not investigated, and the relationship between ghrelin and these adipocytokines were not evaluated.
In conclusion, the results of this study imply that increased ghrelin is closely related to inflammatory pathogenesis and might be used as a surrogate marker of disease activity. Further studies with a large number of patients with UC and CD are needed to test this marker in clinical practice.
References
1. Goh J, O'Morain CA. Review article: nutrition and adult inflammatory bowel disease. Aliment Pharmacol Ther 2003;17:307-320.PMID: 12562443.


2. Bamias G, Nyce MR, De La, Cominelli F. New concepts in the pathophysiology of inflammatory bowel disease. Ann Intern Med 2005;143:895-904.PMID: 16365470.


3. Karmiris K, Koutroubakis IE, Xidakis C, Polychronaki M, Voudouri T, Kouroumalis EA. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm Bowel Dis 2006;12:100-105.PMID: 16432373.


4. Deboer MD. Use of ghrelin as a treatment for inflammatory bowel disease: mechanistic considerations. Int J Pept doi:10.1155/2011/189242. Published online 9 August 2011.


5. DeBoer MD. Emergence of ghrelin as a treatment for cachexia syndromes. Nutrition 2008;24:806-814.PMID: 18725076.


6. Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature 2001;409:194-198.PMID: 11196643.



7. Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 2000;407:908-913.PMID: 11057670.



8. Dixit VD, Schaffer EM, Pyle RS, et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest 2004;114:57-66.PMID: 15232612.



9. Granado M, Priego T, Martin AI, Villanua MA, Lopez-Calderon A. Anti-inflammatory effect of the ghrelin agonist growth hormone-releasing peptide-2 (GHRP-2) in arthritic rats. Am J Physiol Endocrinol Metab 2005;288:E486-E492.PMID: 15507538.


10. Capristo E, Farnetti S, Mingrone G, et al. Reduced plasma ghrelin concentration in celiac disease after gluten-free diet treatment. Scand J Gastroenterol 2005;40:430-436.PMID: 16028437.


11. Suzuki H, Masaoka T, Hosoda H, et al. Helicobacter pylori infection modifies gastric and plasma ghrelin dynamics in Mongolian gerbils. Gut 2004;53:187-194.PMID: 14724148.



12. Peracchi M, Conte D, Terrani C, et al. Circulating ghrelin levels in celiac patients. Am J Gastroenterol 2003;98:2474-2478.PMID: 14638351.


13. Lanzini A, Magni P, Petroni ML, et al. Circulating ghrelin level is increased in coeliac disease as in functional dyspepsia and reverts to normal during gluten-free diet. Aliment Pharmacol Ther 2006;23:907-913.PMID: 16573793.


14. Zhang JV, Ren PG, Avsian-Kretchmer O, et al. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin's effects on food intake. Science 2005;310:996-999.PMID: 16284174.


15. Alexandridis E, Zisimopoulos A, Liratzopoulos N, Katsos I, Manolas K, Kouklakis G. Obestatin/ghrelin ratio: a new activity index in inflammatory bowel diseases. Inflamm Bowel Dis 2009;15:1557-1561.PMID: 19408254.


16. Travis SP, Higgins PD, Orchard T, et al. Review article: defining remission in ulcerative colitis. Aliment Pharmacol Ther 2011;34:113-124.PMID: 21615435.


17. Seo HI, Park DI, Kim TO, et al. The effect of infliximab on patients with ulcerative colitis in Korea. Intest Res 2014;12:214-220.PMID: 25349595.



18. Hwangbo Y, Kim HJ, Shim JJ, et al. Change of circulating leptin, adiponectin, resistin, and visfatin level after treatment of patients with active inflammatory bowel disease. Intest Res 2010;8:151-161.

19. Hosomi S, Oshitani N, Kamata N, et al. Phenotypical and functional study of ghrelin and its receptor in the pathogenesis of Crohn's disease. Inflamm Bowel Dis 2008;14:1205-1213.PMID: 18425803.


20. Karmiris K, Koutroubakis IE. Resistin: another rising biomarker in inflammatory bowel disease? Eur J Gastroenterol Hepatol 2007;19:1035-1037.PMID: 17998823.


21. Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 2006;6:772-783.PMID: 16998510.



22. Karmiris K, Koutroubakis IE, Kouroumalis EA. Leptin, adiponectin, resistin, and ghrelin--implications for inflammatory bowel disease. Mol Nutr Food Res 2008;52:855-866.PMID: 18383234.


23. Gonzalez-Rey E, Chorny A, Delgado M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology 2006;130:1707-1720.PMID: 16697735.


24. De Smet B, Thijs T, Moechars D, et al. Endogenous and exogenous ghrelin enhance the colonic and gastric manifestations of dextran sodium sulphate-induced colitis in mice. Neurogastroenterol Motil 2009;21:59-70.PMID: 18823291.


25. Yamamoto K, Kiyohara T, Murayama Y, et al. Production of adiponectin, an anti-inflammatory protein, in mesenteric adipose tissue in Crohn's disease. Gut 2005;54:789-796.PMID: 15888786.