 |
 |
- Search
| Intest Res > Volume 22(2); 2024 > Article |
|
Abstract
Background/Aims
Methods
Results
ADDITIONAL INFORMATION
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Data Availability Statement
The anonymized data can be obtained from the corresponding author on a reasonable request.
Author Contributions
Conceptualization: Sharma V. Data curation; Formal analysis: Sharma V, Neelam PB. Investigation; Methodology: Neelam PB, Pal R, Sekar A, Bhadada SK, Gupta P, Sharma V. Project administration: Sharma V, Dutta U. Resources: Singh AK, Gupta P, Shah J, Mandavdhare HS, Sharma V, Singh H, Dutta U. Software: Neelam PB. Supervision; Validation: Sharma V, Bhadada SK, Dutta U. Visualization: Neelam PB, Sharma V. Writing - original draft: Neelam PB. Writing - review & editing: Sharma V. Approval of final manuscript: all authors.
Supplementary Material
Supplementary Table 1.
Supplementary Table 2.
Supplementary Table 3.
Supplementary Fig. 1.
Fig. 1.
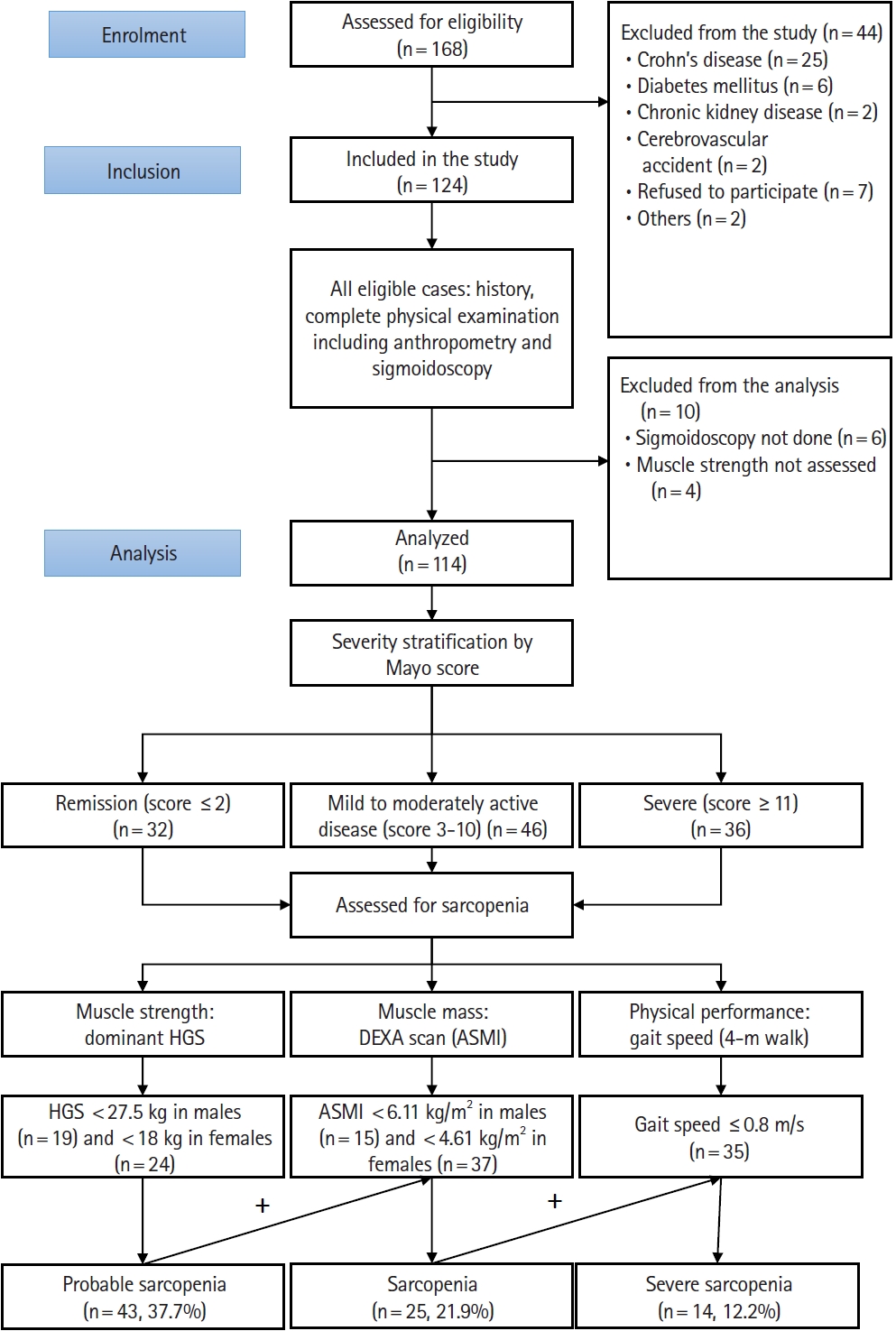
Fig. 2.
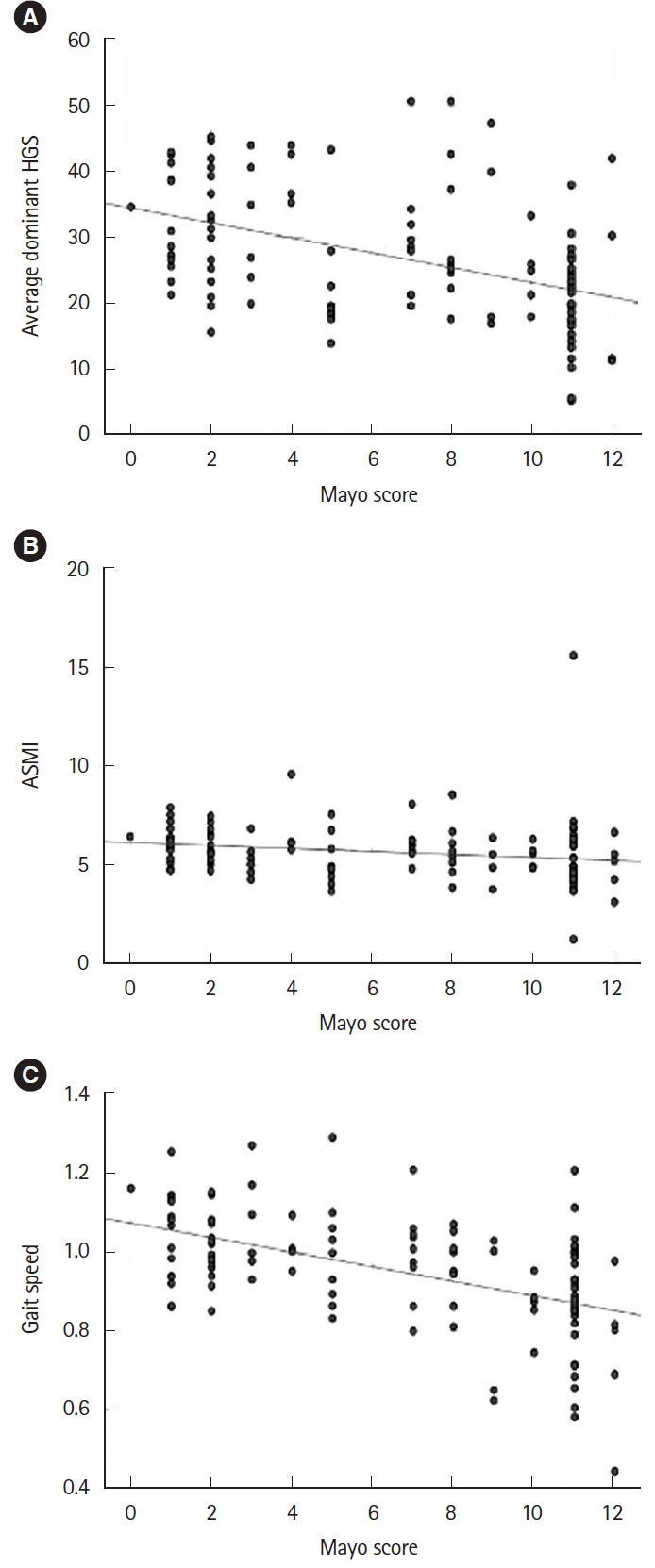
Table 1.
| Baseline variable | No sarcopenia (n = 89) | Sarcopenia (n = 25) | P-value |
|---|---|---|---|
| Male sex | 46 (51.7) | 16 (64.0) | 0.277a |
| Age (yr) | 33.0 (25.8-49.3) | 40.5 (21.3-49.3) | 0.365b |
| Smoking | 9 (10.1) | 4 (16.0) | 0.026a |
| Triceps skinfold thickness (mm) | 7.9 (5.5-11.0) | 5.0 (2.9-8.6) | 0.661b |
| Body mass index (kg/m2) | 21.41 (18.21-24.10) | 17.51 (15.86-17.51) | < 0.001b |
| Disease duration (mo) | 29.0 (17.3-48.0) | 12.0 (2.0-36.0) | 0.048b |
| Disease extentc | 0.108a | ||
| E1 | 9 (11.8) | 0 | |
| E2 | 28 (36.8) | 6 (27.3) | |
| E3 | 39 (51.4) | 16 (72.7) | |
| Hemoglobin (g/dL) | 10.15 (9.35-11.17) | 8.55 (6.87-10.32) | 0.023b |
| Total counts (× 106/L) | 8,300 (6,300-9,725) | 7,600 (6,625-11,525) | 0.764b |
| Neutrophil-lymphocyte ratio | 3.66 (2.29-6.33) | 4.11 (3.40-6.15) | 0.001b |
| Albumin (g/dL) | 3.87 (2.87-4.32) | 3.38 (2.66-3.71) | 0.004b |
| Calcium (mg/dL) | 8.90 (8.66-9.28) | 9.00 (8.67-9.20) | 0.495b |
| Vitamin D (ng/mL) | 15.90 (10.41-28.17) | 15.50 (9.90-26.17) | 0.429b |
| C-reactive protein (mg/L) | 8.09 (1.98-24.70) | 18.72 (12.41-33.51) | 0.004b |
| Total cholesterol (mg/dL) | 115.3 (96.3-147.2) | 143.3 (103.1-173.5) | 0.825b |
| Dominant HGS (kg) | 27.83 (19.16-39.66) | 17.00 (11.66-20.83) | < 0.001b |
| ASMI (kg/m2) | 5.67 (4.89-6.25) | 4.43 (4.11-4.62) | < 0.001b |
| Gait speed (m/s) | 1.00 (0.89-1.06) | 0.89 (0.80-0.96) | 0.007b |
| Mayo score | 8.50 (3.50-11.00) | 11.00 (7.25-11.25) | < 0.001b |
Table 2.
| Disease activity | Remission (n = 32) | Mild-moderately active UC (n = 46) | Severe UC (n = 36) | P-value |
|---|---|---|---|---|
| Probable sarcopenia | 5 (15.6) | 11 (23.9) | 27 (75.0) | < 0.001a |
| Sarcopenia | 2 (6.3) | 6 (13.0) | 17 (47.2) | < 0.001a |
| Severe sarcopenia | 0 | 1 (2.2) | 13 (36.1) | < 0.001a |









