|
 |
- Search
| Intest Res > Volume 21(3); 2023 > Article |
|
See Editorial "Beyond the survey, to the ideal therapy for Asian" in Volume 21 on page 280.
Abstract
Background/Aims
Long-term immunosuppressive therapies used to treat inflammatory bowel disease (IBD) are associated with an increased risk of infections, many of which can be prevented by vaccination. We assessed physicians’ current approaches and clinical practices regarding vaccinations for IBD patients in different Asian countries/regions.
Methods
An internet-based survey was conducted among members of the Asian Organization for Crohn’s and Colitis from September 2020 to November 2020. The questionnaire consisted of 2 parts covering general opinion on the relevance of vaccinations and clinical practice of vaccination.
Results
Overall, 384 Asian medical doctors responded to the survey. The majority of respondents considered it very (57.6%) or sufficiently (39.6%) important to perform vaccinations as recommended by the guidelines. About half of the Asian physicians (52.6%) were usually or always performing vaccinations. The influenza vaccine was the most frequently recommended vaccine for IBD patients. Half of the respondents (51.3%) did not recommend hepatitis A vaccine, especially in China (61.6%) and Japan (93.6%). The diphtheria, tetanus, and pertussis vaccine were never (35.2%) or rarely (29.4%) recommended.
Conclusions
The findings of this survey indicated similarities among countries/regions in terms of the current approaches and practices regarding vaccination of IBD patients; however, there are some differences that might reflect each country’s domestic vaccination guidelines and health insurance particularly with certain vaccines in some countries/regions. Although Asian physicians largely recommend vaccination, more awareness among doctors and Asian consensus regarding differences in IBD vaccination among countries/regions may be required.
Patients with inflammatory bowel disease (IBD) are often treated with long-term immunosuppressive (IS) therapies such as anti-tumor necrosis factor (TNF) drugs and newer drugs such as anti-integrin, anti-interleukin, or Janus kinase inhibitor drugs. Such patients can be at increased risk of infections [1,2]. Several infections including influenza infection, pneumococcal infections, herpes zoster (HZ) infection, or hepatitis B infection in IBD patients treated with IS therapy are potentially preventable through vaccination. Therefore, adequate immunization for vaccine-preventable infectious diseases is currently recommended for all patients with IBD [3]. The vaccination status of the patient should be determined at the time of diagnosis, and recommended vaccinations should be administered [3]. Thus, the American College of Gastroenterology and European Crohn’s and Colitis Organisation developed guidelines for primary care physicians and gastroenterologists to vaccinate IBD patients [4,5]. However, vaccination rates are lower among IBD patients than in the general population [6,7]. In a Western study on 958 patients, only 47.7% of IBD patients received the hepatitis B vaccine, 42.6% received the pneumococcal vaccine, and 34.1% received the hepatitis A vaccine [6]. According to a Canadian survey, vaccination uptake rates were 61.3% for influenza, 10.3% for pneumococcus, 61.0% for hepatitis B, 52.0% for hepatitis A, 26.0% for varicella, 20.7% for meningococcus, 5.3% for HZ, and 11.0% for herpes papilloma virus (females only) [8]. In another Belgian study, only 32% of patients were completely vaccinated according to the guidelines [9]. The immunization program in India is still in the development phase, and the immunization rates of elective vaccines are low [10]. Physicians in various Asian countries/regions may have different approaches and management strategies for vaccination among IBD patients. We aimed to assess current therapeutic approaches and clinical management strategies for vaccination in Asian patients with IBD in the clinical setting using a questionnaire-based survey.
The first survey on Asian doctors’ perspectives towards IBD was conducted in 2014 as one of the programs of the second annual meeting of the Asian Organization for Crohn’s and Colitis (AOCC) [2]. For the follow-up research, the Korean Association for the Study of Intestinal Diseases (KASID) conducted a second survey and presented the results in the 8th annual meeting of AOCC, which was held as a virtual congress in December 2020. The questionnaire for this research was developed in collaboration with the International Academic Exchange Committee, the Scientific Committee, and the IBD research group of KASID; it consisted of 5 main parts: patients’ personal information (8 items), diagnosis of IBD (17 items), treatment of IBD (33 items), infections in IBD (22 items), and vaccinations for IBD (15 items). The IBD research group of KASID designed the questionnaire on vaccinations for IBD. The 15 questions were divided into 2 categories as follows: (1) general opinions on the relevance of vaccinations and (2) clinical practice of vaccination. Details of the questionnaire are shown in the Supplementary Material.
A web-based survey consisting of 95 questions was sent to about 16,000 multinational AOCC members with available email addresses using SurveyMonkey. Online responses were collected between September 16, 2020, and November 13, 2020. The survey was anonymous, and the data were analyzed without revealing the personal details of the doctors. This study reports the subset of data regarding vaccinations for IBD patients obtained from the entire questionnaire. The results of other topics in the questionnaire will be reported elsewhere. The study was approved by the Ethical Committee of Pusan National University Yangsan Hospital (approval number: 05-2023-025). The retrospective data are anonymous and do not involve personal privacy and commercial interests, and the requirement for informed consent was waived.
We used descriptive statistics (frequencies and percentages) to present the data. Respondents could indicate how frequently certain management strategies were used (never 0%- 10%, rarely 10%-30%, sometimes 30%-70%, frequently 70%-90%, and always 90%-100%). The vaccination recommendation data according to the guidelines for IBD patients and the comparison of the effects of suggested vaccinations in patients treated and not treated with immunosuppressants or biologics were analyzed. The collected data were analyzed using SPSS for Windows version 22.0 statistical software (IBM Corp., Armonk, NY, USA). The chi-square test was used for the analysis. The level of statistical significance was set at P=0.05.
Overall, 384 Asian medical doctors (male, 65.9%) responded to the survey. The respondents belonged to various Asian countries/regions (Korea: 110, China: 99, Japan: 93, Taiwan: 20, Hong Kong SAR, China: 10, Vietnam: 8, Indonesia: 7, India: 6, Malaysia: 6, India: 6, Philippines: 4, Pakistan: 3, Australia: 2, Bangladesh: 2, Myanmar: 2, Thailand: 2, Turkey: 2, Egypt: 1, Iraq: 1, Lebanon: 1, Mongolia: 1, New Zealand: 1, Singapore: 1, the United Arab Emirates: 1, and Uzbekistan: 1). Most of the doctors (87.2%, 335/384) were working in academic-affiliated hospitals. Of the total respondents, 211 (54.9%) were gastroenterologists specializing in IBD, 132 (34.4%) were general gastroenterologists, 41 (10.7%) were pediatricians, surgeons, and others. Approximately half of the respondents (46.1%, 177/384) had more than 10 years of clinical experience of caring for patients with IBD, and 40.9% (157/384) were managing between 100 and 500 IBD patients in their clinics (Table 1).
Regarding the importance of performing vaccinations for IBD patients as recommended by the guidelines, most of the respondents considered it to be very important (57.6%, 221/384) or important enough (quite important) (39.6%, 152/384), whereas few (2.9%, 11/384) considered it to be not important. About half of the respondents (57.3%, 220/384) thought that the correct timing of vaccination was at diagnosis of the disease; 15.1% (58/384) and 16.9% (65/384) respondents answered “before starting a treatment with an immunomodulator (IM)” and “before starting a treatment with a biologic,” respectively. In total, 71.6% (275/384) of the respondents prescribing vaccinations for IBD patients were IBD specialists. Regarding the efficacy of immune protection imparted by vaccines in IBD patients treated with immunosuppressants or biologics, 58.6% (225/384) of respondents stated that the efficacy could be reduced. Regarding their general knowledge on vaccinations, 33.3% (128/384) of participants selected sufficient, followed by good (31.8%), insufficient (25.8%), and excellent (9.1%). Regarding the information on the vaccination status of IBD patients, 33.9% (130/384) of the respondents sometimes recorded it and 29.2% (112/384) collected it usually (Fig. 1).
The recommendation of vaccinations, according to the guidelines, to IBD patients was always made by 15.4% (59/384), usually made by 37.2% (143/384), and sometimes made by 30.5% (117/384) of respondents. A subgroup analysis was performed by dividing the “never, rarely, sometimes” and “usually, always” groups. Based on the baseline characteristics, only countries showed a significant difference between the 2 groups (P<0.001). Respondents in the group that responded “usually, always” were 80.9% in Korea, 39.4% in China, 33.3% in Japan, and 52.4% in other countries (Table 2). Influenza, hepatitis B, and pneumococcus vaccines were frequently recommended vaccines in IBD patients regardless of treatment with IS or biologics. Varicella zoster (47.9% vs. 18.5%, P<0.001), measles, mumps and rubella (45.1% vs. 8.1%, P<0.001), and chickenpox (27.6% vs. 7.8%, P<0.001) vaccines are less recommended in IBD patients treated with IS or biologics than in those not treated with IS or biologics (Table 3). The majority of respondents (66.4%, 255/384) recommended the annual influenza vaccine to every IBD patients. Among respondents, 36.2% (139/384) recommended the 5-year pneumococcal vaccine to every IBD patient and 28.9% (111/384) recommended it to those aged over 65 years. The majority of Korean respondents (68.2%, 75/110) recommended pneumococcal vaccine to every IBD patient, compared to 13.1% and 12.9% of the respondents from China and Japan, respectively. The majority of the Japanese respondents (77.4%, 72/93) recommended pneumococcal vaccine to IBD patients aged over 65 years. The vaccine against human papilloma virus (HPV) was recommended by one-third of the respondents (35.9%, 138/384) for all women aged under 26 years, while it was rarely recommended for all patients (2.6%, 10/384). Serologic tests for hepatitis A were not recommended by 30.2% (116/384) respondents. The majority of the respondents (72.0%, 67/93) from Japan never checked the hepatitis A serologic test results, whereas majority of the respondents (65.5%, 72/110) from Korea checked it always or usually. Half of the respondents (51.3%, 197/384) did not recommend the vaccine against hepatitis A virus (HAV), especially in China (61.6%, 61/99) and Japan (93.6%, 87/93). The diphtheria, tetanus, and pertussis vaccine was never (35.2%, 135/384) or rarely (29.4%, 113/384) recommended (Fig. 2).
IBD patients are at higher risk of infection due to treatment with long-term IS therapies [7]. A large study on 140,480 IBD patients reported a higher risk of influenza (incidence rate ratio, 1.54; 95% confidence interval [CI], 1.49-1.63) [11]. A meta-analysis of 14,590 IBD patients showed an elevated risk of any infection (odds ratio, 1.19; 95% CI, 1.10-1.29) and of opportunistic infections (odds ratio, 1.90; 95% CI, 1.21-3.01) [12]. Several infections are potentially preventable though vaccination. Despite clinical guideline recommendations for vaccination in IBD patients, there is insufficient awareness about vaccinations [13,14]. The reasons for the low rates of vaccinations in IBD patients may be the doctor’s lack of awareness, uncertainties about vaccination outcomes, and concerns about potential side effects [8,15-17]. A survey among American gastroenterologists revealed poor knowledge about recommended vaccinations for IBD patients [14]. In one Korean study, gastroenterologists have insufficient knowledge about vaccination of IBD patients [16]. In our survey including Asian physicians, vaccination suggestion rates were higher than those in other Western studies. In this survey, knowledge of vaccination was sufficient, good, and excellent in 74.2% of respondents. Many respondents (44.3%) were usually or always collecting information on vaccination, and about half of the Asian doctors (52.6%) were usually or always performing vaccination. The practicing gastroenterologist should be able to educate patients and counsel them about vaccines considering their safety and effectiveness; they should also inform patients about contraindicated vaccinations in immunosuppressed individuals.
Gastroenterologists treating IBD patients should understand the appropriate use of vaccines. All IBD patients should receive non-live vaccines including influenza vaccine, pneumococcal vaccine, and hepatitis A, hepatitis B, HPV, tetanus, and pertussis vaccines. However, live-attenuated vaccines such as measles, mumps and rubella, varicella, and HZ should be avoided in immunosuppressed patients on high doses of IMs and biologics. IMs should be withheld for a minimum of 2 weeks, preferably for 4 to 6 weeks after live vaccine administration [4,5,18]. Live vaccines should be avoided for at least 3 months after discontinuing treatment with IMs and biologics therapies [19]. Live vaccines could be safely administered in patients undergoing low dose immunosuppression therapy such as those receiving ≤ 20 mg of prednisone for ≤ 14 days, those receiving ≤ 0.4 mg/kg of methotrexate per week, those receiving ≤ 3 mg/kg of azathioprine per day, and those receiving ≤ 1.5 mg/kg of 6-mercaptopurine per day [5]. Patients on monotherapy with thiopurines or biologics may have an adequate response to vaccination, but in patients on combination therapy, there could be reduced response to the vaccine [20-22]. However, there is a report of nearly a 40% and 70% lower chance of achieving an adequate response to vaccinations in patients on IM monotherapy and anti-TNF monotherapy, respectively, than in patients not on IM therapy [20].
There are various international and country-specific guidelines tailored to each country regarding vaccination for IBD patients [1,3,23]. It is important to understand immunization guidelines for IBD patients and convey recommendations to patients. However, in our study, 16.9% (65/384) of respondents never or rarely made such recommendations. According to the guidelines for IBD patients, the recommended rate of vaccinations was significantly different from a country perspective. Respondents in the “usually, always” group were more numerous in Korea than in China, Japan, and other countries. Although influenza vaccination, pneumococcal vaccination, hepatitis B vaccination rates were high, HAV and tetanus/diphtheria (Td) vaccination rates were low. Annual vaccination against influenza was recommended by most respondents (66.4%). IBD patients should receive pneumococcal vaccination with both PCV-13 and PPSV-23 vaccines. Interestingly, 68.2% of the respondents from Korea recommended the 5-year pneumococcal vaccine to every IBD patient, compared to only 13.1% and 12.9% of the respondents from China and Japan, respectively. Vaccination against HPV is recommended to all women aged under 26 years. After checking the immune status, vaccination against HAV and hepatitis B virus should be considered. The survey results of HAV vaccination indicated different approaches among Asian countries/regions. For example, in Japan, the majority of respondents (72.0%) never checked the hepatitis A serology test results and most (51.3%) never recommended vaccination against HAV. The reasons for this relate to the different insurance and domestic vaccination guidelines in each country/region. Our findings suggest that more attention needs to be given to HAV vaccination counselling.
Regarding the tetanus, diphtheria, and pertussis vaccine, a one-time tetanus, diphtheria, and pertussis vaccine and a Td booster every 10 years are recommended. Our findings indicated low rates of vaccination against diphtheria, tetanus, and pertussis (never, 35.2%; rarely, 29.4%). HZ vaccination is recommended in patients aged 50 years and above [5]. IBD patients have an increased risk of HZ than non-IBD patients (incidence rate ratio, 1.68; 95% CI, 1.60-1.76) [24]. Anti-TNF medication, corticosteroids, thiopurines and JAK inhibitors are associated with HZ infection [25,26]. Vaccination against varicella zoster is recommended in those without a clear history of chickenpox or those with no vaccination history [5]. Two vaccines, i.e., live vaccine (ZVL, Zostavax®) and recombinant zoster vaccine (RZV, Shingrix®) are approved [27]. If available, the inactivated vaccine (Shingrix®) should be used. Vaccination against meningococcus and yellow fever should be considered in special situations. Yellow fever is spread by mosquitoes and causes symptoms including fever, chills, headache, backache, and muscle aches. About 15% of people with yellow fever disease develop serious illness that can sometimes be fatal [4]. Individuals traveling to certain parts of South America and Africa are at a risk of yellow fever. There are no data on yellow fever vaccination in patients with IBD under IS therapy. Patients with IBD who are immunosuppressed while traveling to areas where yellow fever is prevalent should consult with a travel medicine or infectious disease specialist prior to travel [5].
This study had several limitations. The first limitation of this study is the response bias, i.e., even if the respondents did not fully understand or were not interested in a question, they had to respond; thus, their answers could be inaccurate. However, owing to the anonymous nature of the survey, respondents might have provided honest answers to the questions. The second limitation is that there was no question in the survey regarding severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) vaccination due to the lack of knowledge about SARS-CoV-2 vaccination at the time of the survey. SARS-CoV-2 vaccination is currently strongly recommended for IBD patients [28]. Nevertheless, our study is the first to present data about vaccination in IBD patients in Asian countries/regions. The response rate to the questionnaire was acceptable and large number of respondents were geographically balanced among the Asian countries/regions.
Asian physicians appear to have enough knowledge on vaccines and performing vaccinations in patients with IBD. Influenza, pneumococcal vaccination, and hepatitis B vaccination rates were high. However, vaccination rates according to guidelines, target of pneumococcal vaccine, hepatitis A and Td vaccine rates were different among countries/regions. It is important to understand immunization guidelines for IBD patients and accordingly convey recommendations, notwithstanding differences between countries/regions regarding health insurance and national vaccination guidelines.
In conclusion, the present survey revealed that current approaches and clinical management of vaccination in IBD patients are mostly similar in Asian countries/regions; however, there are some differences among vaccines in some countries/regions. Although Asian physicians largely recommend vaccination, more awareness among doctors and Asian consensus regarding differences in IBD vaccination among countries/regions may be required.
ADDITIONAL INFORMATION
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Supplementary Material
Supplementary materials are available at the Intestinal Research website (https://www.irjournal.org).
Supplementary Material.
Detailed questions used in the current survey
Fig. 1.
Set of question 1. (A-F) General opinions on the relevance of vaccinations. IBD, inflammatory bowel disease; IM, immunomodulator.
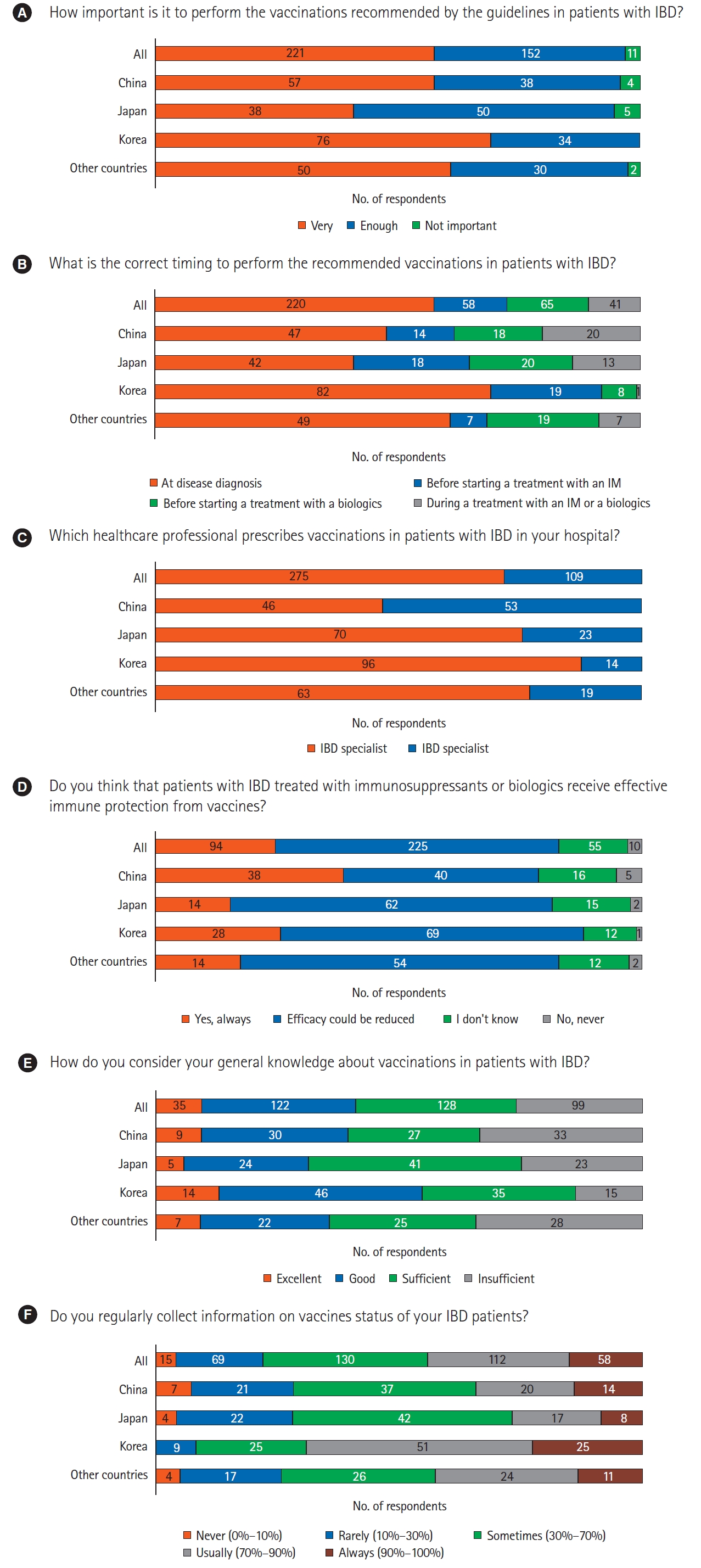
Fig. 2.
Set of question 2. (A-I) Clinical practice of vaccination strategies. IBD, inflammatory bowel disease; HAV, hepatitis A virus; HBV, hepatitis B virus; HPV, human papilloma virus; VZV, varicella zoster virus; DTP, diphtheria, tetanus, and pertussis; MMR, measles, mumps and rubella.
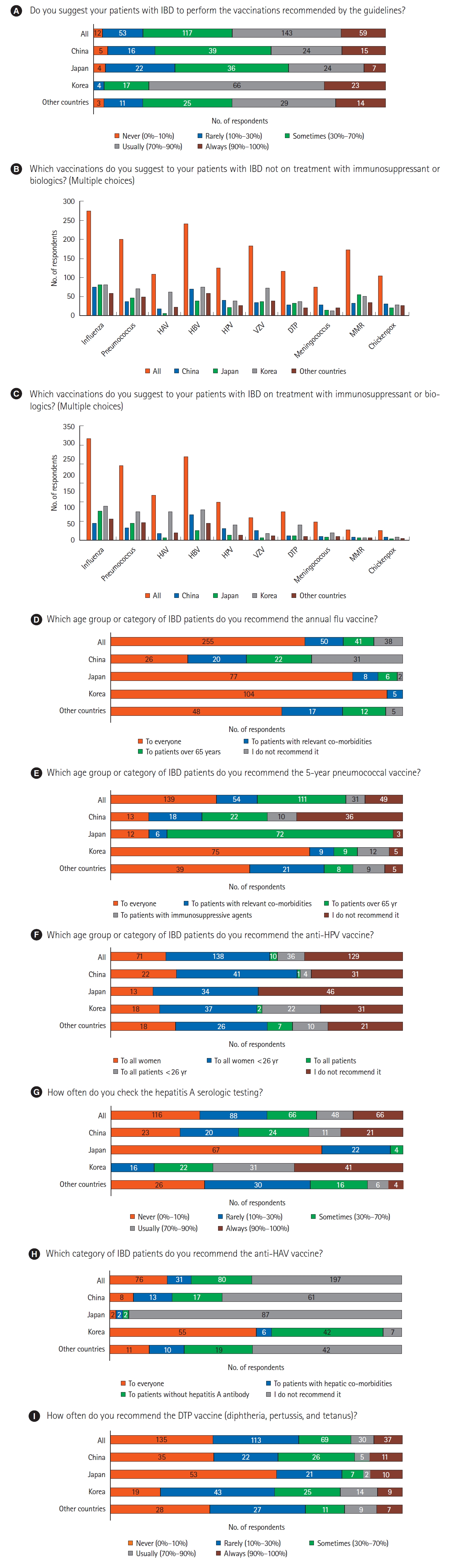
Table 1.
Baseline Characteristics of the Survey Respondents According to Countries/Regions
Table 2.
Characteristics of the Survey on the Recommendation of Vaccinations According to the Guidelines for IBD Patients
Table 3.
Comparison of Suggestion of Vaccinations Not on Treatment and on Treatment with Immunosuppressant or Biologics
REFERENCES
1. Kochar B, Herfarth HH. Vaccinations in adult patients with inflammatory bowel diseases in the West. Inflamm Intest Dis 2018;3:11-15.




2. Kim ES, Chen M, Lee J, Lee CK, Kim YS. Diagnosis of inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 2(nd) Asian Organization for Crohn’s and Colitis (AOCC) meeting in Seoul. Intest Res 2016;14:224-230.



3. Manser CN, Maillard MH, Rogler G, et al. Vaccination in patients with inflammatory bowel diseases. Digestion 2020;101 Suppl 1(Suppl 1):58-68.




4. Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis 2014;8:443-468.


5. Farraye FA, Melmed GY, Lichtenstein GR, Kane SV. ACG clinical guideline: preventive care in inflammatory bowel disease. Am J Gastroenterol 2017;112:241-258.



6. Wasan SK, Calderwood AH, Long MD, Kappelman MD, Sandler RS, Farraye FA. Immunization rates and vaccine beliefs among patients with inflammatory bowel disease: an opportunity for improvement. Inflamm Bowel Dis 2014;20:246-250.

7. Melmed GY, Ippoliti AF, Papadakis KA, et al. Patients with inflammatory bowel disease are at risk for vaccine-preventable illnesses. Am J Gastroenterol 2006;101:1834-1840.


8. Malhi G, Rumman A, Thanabalan R, et al. Vaccination in inflammatory bowel disease patients: attitudes, knowledge, and uptake. J Crohns Colitis 2015;9:439-444.


9. Coenen S, Weyts E, Jorissen C, et al. Effects of education and information on vaccination behavior in patients with inflammatory bowel disease. Inflamm Bowel Dis 2017;23:318-324.


10. Manthiram K, Blood EA, Kuppuswamy V, et al. Predictors of optional immunization uptake in an urban south Indian population. Vaccine 2014;32:3417-3423.


11. Tinsley A, Navabi S, Williams ED, et al. Increased risk of influenza and influenza-related complications among 140,480 patients with inflammatory bowel disease. Inflamm Bowel Dis 2019;25:369-376.


12. Bonovas S, Minozzi S, Lytras T, et al. Risk of malignancies using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and metaanalysis. Expert Opin Drug Saf 2016;15(sup1):35-54.

13. Narula N, Dhillon AS, Chauhan U, Marshall JK. An audit of influenza vaccination status in adults with inflammatory bowel disease. Can J Gastroenterol 2012;26:593-596.




14. Wasan SK, Coukos JA, Farraye FA. Vaccinating the inflammatory bowel disease patient: deficiencies in gastroenterologists knowledge. Inflamm Bowel Dis 2011;17:2536-2540.


15. Yeung JH, Goodman KJ, Fedorak RN. Inadequate knowledge of immunization guidelines: a missed opportunity for preventing infection in immunocompromised IBD patients. Inflamm Bowel Dis 2012;18:34-40.


16. Jung YS, Park JH, Kim HJ, et al. Insufficient knowledge of Korean gastroenterologists regarding the vaccination of patients with inflammatory bowel disease. Gut Liver 2014;8:242-247.



17. Gupta A, Macrae FA, Gibson PR. Vaccination and screening for infections in patients with inflammatory bowel disease: a survey of Australian gastroenterologists. Intern Med J 2011;41:462-467.


19. Reich J, Wasan S, Farraye FA. Vaccinating patients with inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2016;12:540-546.


20. Nguyen DL, Nguyen ET, Bechtold ML. Effect of immunosuppressive therapies for the treatment of inflammatory bowel disease on response to routine vaccinations: a meta-analysis. Dig Dis Sci 2015;60:2446-2453.



21. Gelinck LB, van der Bijl AE, Beyer WE, et al. The effect of antitumour necrosis factor alpha treatment on the antibody response to influenza vaccination. Ann Rheum Dis 2008;67:713-716.

22. Dotan I, Werner L, Vigodman S, et al. Normal response to vaccines in inflammatory bowel disease patients treated with thiopurines. Inflamm Bowel Dis 2012;18:261-268.



23. Lee YJ, Kim ES. Vaccination strategies for Korean patients with inflammatory bowel disease. Korean J Intern Med 2022;37:920-930.




24. Long MD, Martin C, Sandler RS, Kappelman MD. Increased risk of herpes zoster among 108 604 patients with inflammatory bowel disease. Aliment Pharmacol Ther 2013;37:420-429.



25. Winthrop KL, Melmed GY, Vermeire S, et al. Herpes zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflamm Bowel Dis 2018;24:2258-2265.



26. Khan N, Patel D, Trivedi C, et al. Overall and comparative risk of herpes zoster with pharmacotherapy for inflammatory bowel diseases: a nationwide cohort study. Clin Gastroenterol Hepatol 2018;16:1919-1927.


27. Harpaz R, Ortega-Sanchez IR, Seward JF, Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008;57(RR-5):1-30.