Durability and outcomes of fecal microbiota transplantation for recurrent Clostridioides difficile infection in patients with moderate to severe inflammatory bowel disease
-
Raseen Tariq
 , Edward V Loftus
, Edward V Loftus , Darrell Pardi
, Darrell Pardi , Sahil Khanna
, Sahil Khanna
- Received August 18, 2023 Revised September 25, 2023 Accepted November 1, 2023
- Clostridioides difficile infection (CDI) is not only common in the nosocomial setting, but the incidence, severity, and recurrence rates have increased in the community [1]. Patients with inflammatory bowel disease (IBD) may develop CDI without traditional risk factors, and have higher rates of needing IBD therapy escalation, higher likelihood of failing IBD therapy, increased risk of subsequent IBD flare and higher rates of surgery. The risk of recurrent CDI (rCDI) and colectomy are also higher in IBD patients compared to non-IBD patients with CDI [2].Fecal microbiota transplantation (FMT) is an effective therapy for preventing rCDI and is a recommended option in multiple guidelines based on clinical trials and case series [3]. Most clinical trials evaluating the efficacy of FMT for rCDI exclude IBD patients [4]. Based on retrospective studies, FMT is safe and effective in IBD patients with rCDI, and a meta-analysis suggests about a 25% chance of a new IBD flare after FMT [5]. In an open-label prospective cohort study, 49 patients with IBD were treated with FMT for rCDI with a 10.1% failure rate at 8-week follow-up, and only 1 patient had a new IBD flare after FMT. Patients had varying levels of disease activity with 48.5% of the ulcerative colitis (UC) patients having mild activity (partial Mayo score < 4) [6]. In another study of 145 IBD patients, the overall efficacy was 80% at a median follow-up of 9 months, and 57% of patients were either in clinical remission or had mild IBD at the time of FMT [7].There remains a need to assess the efficacy and safety of FMT in patients with moderate to severe colonic IBD, as clinicians may be hesitant to perform an FMT due to significant inflammation at the time of FMT and concern for risk of worsening IBD after FMT. Most of the prior studies have focused on assessing efficacy of FMT within 8 weeks post-procedure, with limited data on durability of response beyond the immediate post-treatment period. The durability of FMT refers to the ability to sustain the primary response (lack of rCDI) within 1 year of FMT despite an ongoing risk factor for dysbiosis such as active IBD [8]. The aim of our study was to assess the effect of FMT for rCDI on the clinical course in patients with moderate to severe IBD. We also assessed the long-term durability of FMT at 1 year and rates of colectomy until last follow-up.We conducted a retrospective study of adults with endoscopically moderate to severe colonic IBD undergoing FMT via colonoscopy for rCDI at Mayo Clinic, Rochester, MN from September 2012 to June 2022. We included patients with rCDI (2 or more recurrences) with underlying colonic IBD. rCDI was defined as 3rd or greater CDI episode proven by a positive C. difficile stool assay in the presence of diarrhea, and previous antibiotic treatment for rCDI. Our donor screening protocol and patient screening processes have been previously published. Donors are usually anonymous. We use an iterative protocol to screen donors for several infectious and noninfectious conditions. A total of 50 g of stool is used to prepare the product in a final volume of 250 mL of 90% normal saline and 10% glycerol. The freeze-thawed product is instilled via a colonoscope into the cecum [9]. We defined moderate to severe disease based on clinical symptoms and endoscopic findings at the time of FMT. Endoscopic IBD severity was captured at the time of FMT utilizing endoscopic grading systems (Simple Endoscopic Score for Crohn’s Disease and the Mayo endoscopic subscore). The study was approved by the Institutional Review Board at Mayo Clinic (IRB #15-005818). The written informed consent was waived.Data collected included demographics, IBD and CDI history, treatments for IBD, and post-FMT outcomes. The presence of an IBD flare at the time of FMT was determined based on the clinical judgment of the clinician caring for the patient. We recorded the presence of IBD flares (worsening IBD symptoms with or without IBD therapy escalation) at the time of FMT and if patients had an IBD flare after FMT. IBD flare after FMT was defined as ongoing symptoms with negative CDI test after FMT.The primary outcome was the durability of FMT defined as no CDI recurrence at 1 year follow-up. Secondary outcomes were adverse events from FMT and IBD therapy escalation after FMT. We also calculated the rate of colectomy until last follow-up.Data analysis included descriptive statistics, t-tests for normally distributed continuous variables, nonparametric tests for skewed variables, and chi-square test or analysis of variance for categorical variables. P values ≤ 0.05 were considered significant. Kaplan-Meier survival analysis was used to calculate the proportion of patients with durable response and proportion of patients who had colectomy. Analyses were done using BlueSky Statistics software (BlueSky Statistics LLC, Chicago, IL, USA).Overall, 53 patients with moderate to severe colonic IBD were included (median age, 41 years; range, 19–84 years; 39% of females). Thirty-six (68%) had UC, 14 (26%) had ileocolonic Crohn’s disease and 3 (6%) had indeterminate colitis. The median duration of IBD was 6 years (range, 1–50 years). Endoscopic severity at the time of FMT was moderate in 20 (37.7%) and severe in 33 (62.3%) patients. Median number of CDI episodes prior to FMT was 3 (range, 3–15) (Table 1).At the time of FMT, 75% of patients (n = 40) had an ongoing IBD flare. Of those 40 patients, 80% continued to flare after FMT. After FMT, 79.8% had no change in their IBD status, 11.3% had symptom improvement, 9% had a new flare, and 84% of patients had planned escalation of IBD therapy (Supplementary Fig. 1).At 1 year follow-up, rCDI occurred in 12 patients, suggesting an overall durable response of 76.8% (95% confidence interval [CI], 66.2%–89.2%) (Fig. 1A). There were no significant associations between rCDI after FMT and clinical variables including prior antibiotics for CDI, ileal or colonic disease, IBD severity or subtype, steroids, 5-aminosalicylates, immunomodulators, or biologic use before or at the time of FMT; IBD flare after FMT; or escalation of IBD therapy after FMT. No serious adverse events were seen in 46 patients, while 7 had an IBD flare within 4 weeks, 2 of whom needed an emergency room visit and 1 required colectomy. Of the 12 patients who had recurrence after first FMT, 4 (33%) underwent repeat FMT and 8 were managed with antibiotics. Two patients who underwent repeat FMT had recurrence and were managed with indefinite daily vancomycin.During an extended follow-up period, despite intensified IBD treatment, 15 patients, representing a 38% probability (95% CI, 20%–52%), underwent colectomy due to medically unresponsive IBD over a span of 8.2 years (Fig. 1B). On univariate analysis, no difference was noted in patients with or without new CDI episode and risk of colectomy hazard ratio 2.22 (95% CI, 0.78–6.28; P= 0.13).In our study, three-fourths of IBD patients with rCDI had a durable response to FMT for up to 1 year despite having moderate to severe IBD disease activity. None of the IBD-related factors predicted recurrence after FMT. FMT had no beneficial effect on the course of IBD, with more than 80% of patients requiring IBD therapy escalation for IBD flare after clearance of CDI. We hypothesize that inflammation in the colon may lead to decreased microbial engraftment and lead to decreased response to FMT. However, despite moderate to severe inflammation, FMT had a durable response at 1 year in most patients and was better tolerated than previously reported in retrospective studies [5].There have been concerns regarding de novo IBD flares after FMT, with rates as high as 25% in retrospective studies [5]; however, the only prospective trial evaluating the risk for potential IBD flare post-FMT in CDI-IBD patients showed a marginally increased risk of de novo IBD flare (4%) [6]. One recent multicenter study of CDI-IBD patients from Europe found that the overall resolution rate at 8 weeks was 71% with one FMT, only 5% developed a new IBD flare, and about half the patients in their cohort had remission at the time of rCDI [10]. In our study, IBD disease status remained stable in most of the patients, and few patients (13%) had a new IBD flare after FMT.We found that a significant number of patients needed colectomy due to their severe IBD, despite IBD therapy escalation. These findings suggest that earlier use of FMT for CDI for early eradication and subsequently early IBD therapy escalation may be beneficial, although more studies are needed.The study has several limitations. While this study provides insights durability of FMT for rCDI, the limited sample size and the absence of data on the microbiome and key markers like fecal calprotectin, C-reactive protein, and erythrocyte sedimentation rate limits its comprehensive applicability. Another limitation of the study was that diagnosis of CDI was based on the clinical judgment of physicians and positive CDI testing, because no objective method can distinguish an IBD flare from CDI colonization.In conclusion, our study shows that FMT is durable and safe in moderate to severe IBD. Our results are useful for clinicians weighing the risks and benefits of using FMT for CDI in this patient population. More research is needed to study the early use of FMT and identifying patients needing early therapy escalation to prevent IBD flare and colectomy.
- ADDITIONAL INFORMATION
- ADDITIONAL INFORMATION
-
Funding Source
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (Grant/Award Number: DK07198).
Conflict of Interest
Khanna S receives research support from Rebiotix/Ferring, Vedanta, Finch, Seres, and Pfizer and serves as a consultant for ProbioTech, Takeda, Niche, and Immuron. Pardi D has grant funding from Pfizer, Vedanta, Seres, Finch, Applied Molecular Transport, and Takeda and has consulted for Vedanta, Seres, AbbVie, Immunic, and Otsuka. Loftus EV Jr has received consulting fees from AbbVie, Amgen, Arena, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, Genentech, Gilead, Gossamer Bio, Iterative Scopes, Janssen, Ono Pharma, Pfizer, Scipher Medicine, Sun Pharma, Takeda. Except for that, no potential conflict of interest relevant to this article was reported.
Supplementary Material
Supplementary Material
Supplementary materials are available at the Intestinal Research website (https://www.irjournal.org).ir-2023-00100-Supplementary-Fig-1.pdfSupplementary Fig. 1.
Flowchart depicting the patient cohort characteristics, diagnosis breakdown, disease duration and severity, response to fecal microbiota transplant and long-term outcomes. IBD, inflammatory bowel disease; CDI, Clostridioides difficile infection; FMT, fecal microbiota transplantation.
Fig. 1.
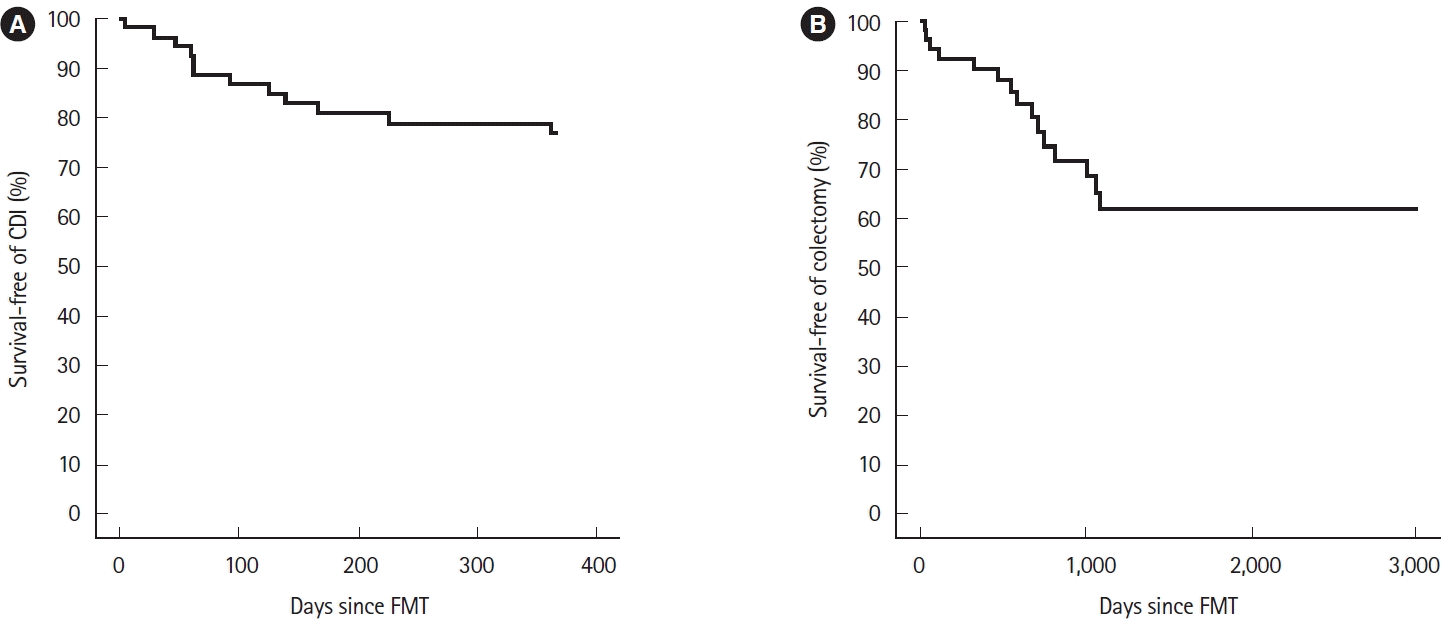
Table 1.
- REFERENCES
- REFERENCES
- 1. Ananthakrishnan AN. Clostridium difficile infection: epidemiology, risk factors and management. Nat Rev Gastroenterol Hepatol 2011;8:17–26.
[Article] [PubMed]2. Khanna S, Shin A, Kelly CP. Management of Clostridium difficile infection in inflammatory bowel disease: expert review from the clinical practice updates Committee of the AGA Institute. Clin Gastroenterol Hepatol 2017;15:166–174.
[Article] [PubMed]3. Kelly CR, Fischer M, Allegretti JR, et al. ACG clinical guidelines: prevention, diagnosis, and treatment of Clostridioides difficile infections. Am J Gastroenterol 2021;116:1124–1147.
[Article] [PubMed]4. Tariq R, Pardi DS, Bartlett MG, Khanna S. Low cure rates in controlled trials of fecal microbiota transplantation for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Clin Infect Dis 2019;68:1351–1358.
[Article] [PubMed]5. Tariq R, Syed T, Yadav D, et al. Outcomes of fecal microbiota transplantation for C. difficile infection in inflammatory bowel disease: a systematic review and meta-analysis. J Clin Gastroenterol 2023;57:285–293.
[PubMed]6. Allegretti JR, Kelly CR, Grinspan A, et al. Inflammatory bowel disease outcomes following fecal microbiota transplantation for recurrent C. difficile infection. Inflamm Bowel Dis 2021;27:1371–1378.
[Article] [PubMed] [PMC]7. Tariq R, Disbrow MB, Dibaise JK, et al. Efficacy of fecal microbiota transplantation for recurrent C. difficile infection in inflammatory bowel disease. Inflamm Bowel Dis 2020;26:1415–1420.
[Article] [PubMed]8. Saha S, Mara K, Pardi DS, Khanna S. Durability of response to fecal microbiota transplantation after exposure to risk factors for recurrence in patients with Clostridioides difficile infection. Clin Infect Dis 2021;73:e1706–e1712.
[Article] [PubMed] [PMC]