Rates of metachronous adenoma after curative resection for left-sided or right-sided colon cancer
Article information
Abstract
Background/Aims
We determined the rates of metachronous colorectal neoplasm in colorectal cancer (CRC) patients after resection for right (R)-sided or left (L)-sided cancer.
Methods
Consecutive CRC patients who had undergone surgical resection for curative intent in our hospital between 2001 and 2004 were identified. R-sided colonic cancers refer to cancer proximal to splenic flexure whereas L-sided cancers include rectal cancers. Patients were included only if they had a clearing colonoscopy performed either before or within 6 months after the operation. Findings of surveillance colonoscopy performed up to 5 years after colonic resection were included in the analysis.
Results
Eight hundred and sixty-three CRC patients underwent curative surgical resection during the study period. Three hundred and twenty-seven patients (107 R-sided and 220 L-sided) fulfilled the inclusion criteria and had at least 1 postoperative surveillance colonoscopy performed. The proportion of patients who had polyp and adenoma on surveillance colonoscopy was significantly higher among patients with L-sided than R-sided cancers (polyps: 30.9% vs. 19.6%, P=0.03; adenomas: 25.5% vs. 13.1%, P=0.01). The mean number of adenoma per patient on surveillance colonoscopy was also higher for patients with L-sided than R-sided tumors (0.52; 95% confidence interval [CI], 0.37–0.68 vs. 0.22; 95% CI, 0.08–0.35; P<0.01). Multivariate analysis showed that L-sided cancers, age, male gender and longer follow-up were independent predictors of adenoma detection on surveillance colonoscopy.
Conclusions
Patients with Lsided cancer had a higher rate of metachronous polyps and adenoma than those with R-sided cancer on surveillance colonoscopy.
INTRODUCTION
Surveillance colonoscopy plays a pivotal role in monitoring of colorectal cancer (CRC) patients after surgical resection, which enables early detection of recurrent cancer as well as metachronous lesions such as polyp or adenoma. Regular surveillance colonoscopy together with other surveillance strategies can detect recurrence at earlier stage and improve survival of CRC patients [1,2].
Although the majority of CRCs develop through the conventional adenoma-carcinoma sequence [3,4], there are a certain proportion of right (R)-sided colon cancers that progress through the serrated neoplastic pathway. In view of the diversity in molecular pathogenesis of CRC, it is highly plausible that the rates of metachronous neoplasms in patients after curative resection for R- or left (L)-sided colon cancers are different.
Studies focusing on the development of pre-cancerous lesions after resection of CRC showed that advanced age and the presence of synchronous neoplasm were associated with development of metachronous neoplasms [5-7]. Although previous studies have evaluated the risk factors for development of metachronous CRC [8-11], there was a paucity of data on the association between the sites of primary tumor and subsequent risk of metachronous adenoma or polyp. Fuccio et al. [12] showed that patients with L-sided primary tumor had a higher risk of metachronous adenoma after surgical resection, suggesting a different rate of metachronous lesions development. However, patients with rectal carcinoma, who are known to be at risk of local recurrence, were not included in their study and the analysis was also limited to the second surveillance colonoscopy only. Current guidelines on surveillance after colorectal resection recommend surveillance colonoscopy to be performed 1 year after surgical resection, and then repeat at 3 to 5 years [13-16]. As yet, patients’ factors, particularly with regards to primary tumor location, were not taken into account when considering the optimal surveillance interval.
In this study, we determined the risk of subsequent adenoma and polyp development in patients after curative resection for L- or R-sided cancer. We also considered the potential confounding effects of age, sex, family history of CRC, aspirin or statin use, stage and site of tumor as well as the duration of follow-up period on the rate of metachronous neoplasms. Our finding may help to tailor the best surveillance strategy for CRC patients according to the site of primary tumor.
METHODS
1. Patients
This is a retrospective cohort study performed in the Queen Mary Hospital of Hong Kong, which is the regional hospital of the Hong Kong West Cluster of the Hospital Authority as well as the teaching hospital of the University of Hong Kong. We retrieved all CRC patients who had undergone curative resection in the department of surgery between January 2001 and December 2004. We only included patients who had a clearing colonoscopy with all polyps removed either before surgery or within 6 months after the operation to ensure the lesions detected on surveillance colonoscopy were metachronous but not previously missed lesions. Patients who had incomplete preoperative colonoscopy due to obstructing tumor but with all the proximal segments removed by surgery were also included in the analysis. Exclusion criteria included patients with (1) familial CRC syndrome including familial adenomatous polyposis syndrome and hereditary non-polyposis colorectal cancer syndrome; (2) IBD; (3) previous subtotal or total colectomy; (4) synchronous cancers at baseline; and (5) baseline cancer being a metachronous or recurrent cancer. All clinical information of individual patient was retrieved from the electronic patient record system of the Hospital Authority of Hong Kong, which is a centralized computer database used in all local public hospitals. The database included all operative and endoscopy records, histopathology and imaging findings, laboratory results, hospital discharge summary and outpatient consultation notes as well as prescription and dispensing records [17]. For each patient, the following information were retrieved including baseline demographics, family history of CRC, the stage and site of the primary tumor, the date and type of operation, use of aspirin and statin, all colonoscopy and sigmoidoscopy findings and their histopathology results.
Patients who had CRC in their first-degree relatives were considered to have positive family history. Patients who were on aspirin or statin at the time of operation and any time within 5 years after resection were considered to be aspirin and statin users, respectively. The stage of CRC was reported according to the American Joint Committee on Cancer Staging for CRC (the 6th edition) [18]. We included all colonoscopies and sigmoidoscopies performed from 6 to 60 months after surgical resection. All procedures were performed by experienced gastroenterologists, colorectal surgeons or trainees under supervision. For all colonoscopies, we recorded the date of procedure, the quality of bowel preparation and details of the lesions identified.
The study was approved by the Institutional Review Board (IRB No. UW 16-458) of the University of Hong Kong and the West Cluster of Hospital Authority of Hong Kong. As this is a retrospective cohort study, informed consent from individual patient could not be obtained and was waived by the IRB.
2. Definition
Advanced adenoma was defined as adenoma with high-grade dysplasia, villous component or of size ≥1 cm [19]. Serrated lesion was classified according to the World Health Organization (WHO) classification [3,20] and included traditional serrated adenoma, sessile serrated adenoma with or without cytological dysplasia, and hyperplastic polyp. In this study, recurrent cancer was defined as cancer diagnosed at or near the anastomosis. Metachronous cancer was defined as cancer diagnosed at a site other than those at or near the anastomosis. For patients who developed recurrent CRC or metachronous CRC, the information was censored from the time when recurrent or metachronous CRC was diagnosed. The quality of bowel preparation was rated according to Aronchick et al. [21]
3. Outcomes
In this study, patients were categorized into 2 groups according to the sites of index cancer. Patients with carcinoma of caecum, ascending colon, hepatic flexure, transverse colon and splenic flexure were considered as R-sided cancer. Those who had carcinoma of descending colon, sigmoid colon, rectosigmoid region and rectum were considered as L-sided cancer.
The primary outcome was the proportion of patients with at least 1 polyp or adenoma on surveillance endoscopies in each group. Secondary outcomes included the mean number of polyp/adenoma per patient. The association of the following factors with adenoma detection on surveillance colonoscopy was evaluated including age, sex, family history of CRC, aspirin use, statin use, stage of cancer, site of cancer, quality of bowel preparation during clearing colonoscopy, number of surveillance colonoscopy and time to last surveillance colonoscopy. Subgroup analysis was performed after exclusion of patients with carcinoma of rectosigmoid region and rectum as well as those with family history of CRC.
4. Statistical Analyses
Categorical variables were reported as proportions. Continuous variables were reported as mean (with 95% CI) or median (with range) where appropriate. Comparison of continuous variables was performed using independent t-test, one-way ANOVA test or Mann-Whitney U-test. Categorical variables were compared using Pearson chi-square test or Fisher exact test. Logistic regression model was used to estimate ORs and 95% CI of parameters to predict adenoma detection on surveillance colonoscopy. The multivariable logistic regression model included the following variables: age, sex, number of surveillance colonoscopy, time to last surveillance colonoscopy and site of index CRC. All statistical analyses were performed using SPSS version 20.0 (IBM Corp., Armonk, NY, USA). A two-sided P-value of <0.05 was considered statistically significant.
RESULTS
1. Patients’ Characteristics
Between January 2001 and December 2004, 863 patients had undergone curative resection for CRC in Queen Mary Hospital and 390 patients were excluded for reasons as listed in Fig. 1. Among the remaining 473 patients, 327 patients had at least 1 surveillance colonoscopy after curative resection and were included in this analysis. The baseline characteristics of patients with at least 1 surveillance colonoscopy were listed in Table 1. The quality of bowel preparation of the clearing colonoscopy was recorded in 261 patients and 234 patients (89.7%) had their bowel preparation rated as good or fair.
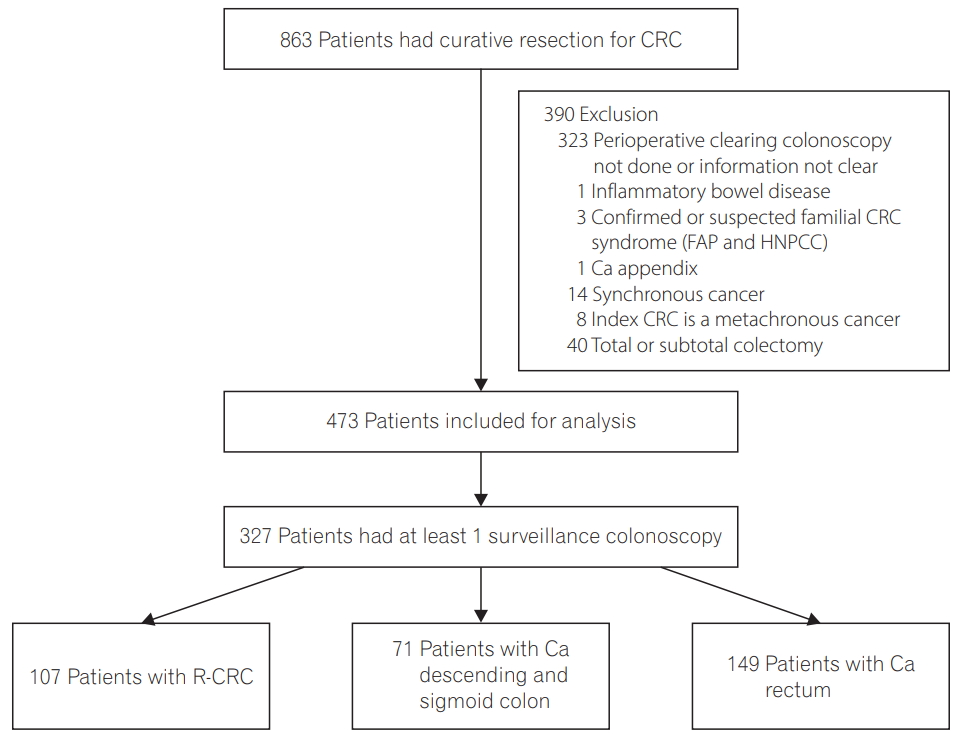
Study flowchart. CRC, colorectal cancer; FAP, familial adenomatous polyposis syndrome; HNPCC, hereditary nonpolyposis colorectal cancer syndrome; Ca, cancer; R-CRC, right-sided colorectal cancer.
2. Surveillance Colonoscopy Findings
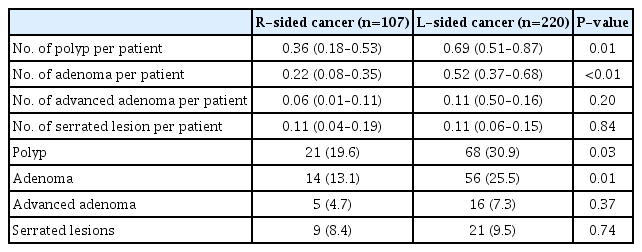
A total of 474 surveillance colonoscopies were performed and the mean number of colonoscopies per patient was 1.45 (95% CI, 1.38–1.52). The number of colonoscopy performed for each group was listed in Table 1. A total of 190 polyps were detected on surveillance colonoscopy. The total number of adenoma, advanced adenoma and serrated lesions were 138, 29, and 35 respectively. The distribution of colonic adenoma in the 2 groups was shown in Fig. 2.
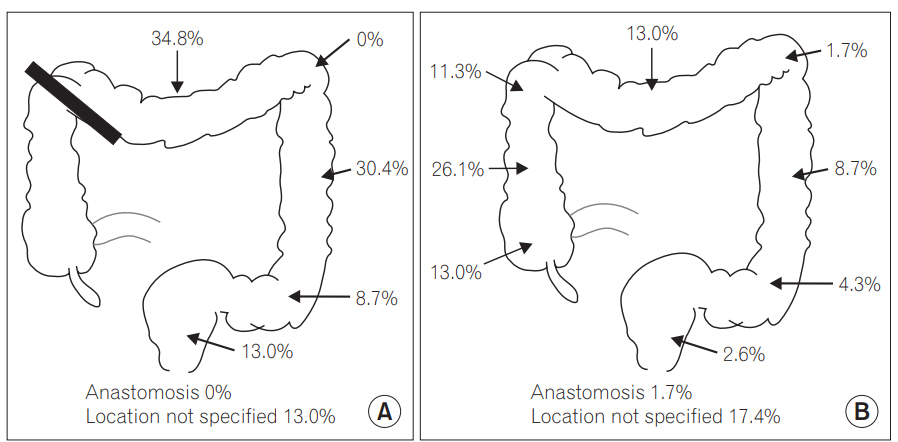
Location of adenoma detected on surveillance colonoscopy. (A) Right-sided cancer: segment proximal to thick black line was removed by surgery, (B) left-sided cancer including rectal cancer.
The cumulative numbers of colonic lesions detected in each group were shown in Table 2. Both the proportions of patients with polyp or adenoma detected on surveillance colonoscopy were significantly higher in patients with L-sided cancer as compared to patients with R-sided cancer (polyps: 30.9% vs. 19.6%, P=0.03 and adenoma: 25.5% vs. 13.1%, P=0.01). Moreover, the mean number of polyp and adenoma on surveillance colonoscopy were significantly higher among patients with L-sided than patients with R-sided cancer (polyp: 0.69; 95% CI, 0.51–0.87 vs. 0.36; 95% CI, 0.18–0.53; P=0.01 and adenoma 0.52; 95% CI, 0.37–0.68 vs. 0.22; 95% CI, 0.08–0.35, P<0.01).
However, there was no significant difference in the prevalence and number of advanced adenoma and serrated lesions between the 2 groups. Five patients with R-sided cancer (4.7%) and 2 patients with L-sided cancer (0.9%) had metachronous cancer detected on surveillance at a median time of 36 months (range, 11–49 months) (P =0.03).
3. Subgroup Analysis
While rectal cancer patients have higher chance of local recurrence [16], we performed subgroup analysis to exclude patients with cancer of rectum and rectosigmoid junction. As shown in Table 3, the differences in the detection rates of polyp and adenoma on surveillance colonoscopy between patients with R- and L-sided cancer after excluding rectal cancer were even more prominent. Fig. 3 showed the proportion of patients with different colonic lesions found on surveillance in the 3 groups of cancer patients according to primary tumor locations. The proportions of patients with metachronous polyp or adenoma was highest among patients with baseline L-sided cancer (P <0.01) but were comparable between patients with baseline R-sided cancer and rectal cancer.
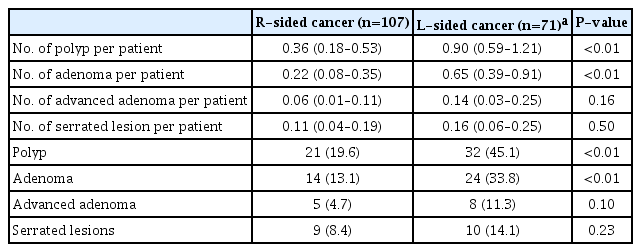
Number of Polyp, Adenoma, Advanced Adenoma and Serrated Lesions on Surveillance Colonoscopy after Excluding Patients with Carcinoma of Rectum and Rectosigmoid Junction
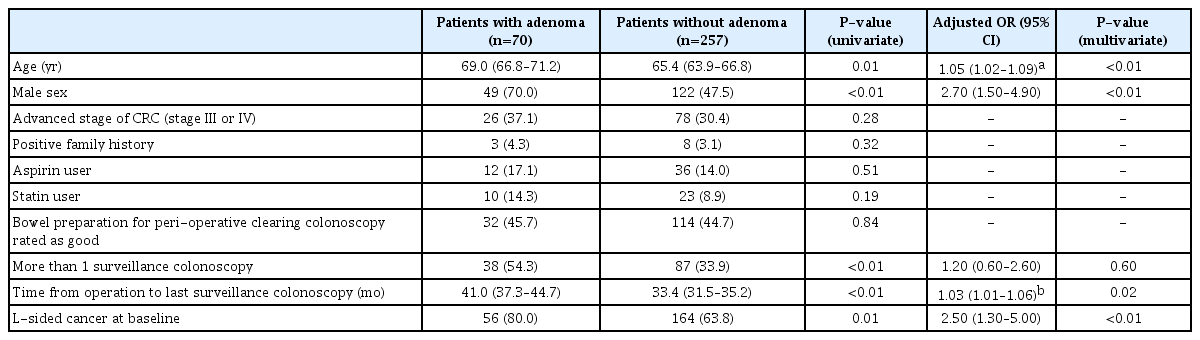
4. Risk of Metachronous Adenoma
Table 4 showed the univariate and multivariate analysis of factors associated with metachronous adenoma on surveillance colonoscopy. Multivariate analysis showed that Lsided cancer (OR, 2.5; 95% CI, 1.3–5.0; P <0.01), increasing age (OR, 1.05; 95% CI, 1.02–1.09; P <0.01), men (OR, 2.7; 95% CI, 1.5–4.9; P <0.01) and longer surveillance period (OR, 1.03; 95% CI, 1.01–1.06; P <0.01) were independent factors associated with metachronous adenoma (Table 4).
DISCUSSION
Our study showed that the rates of metachronous polyps or adenoma were significantly higher in patients with L-sided than R-sided cancer. The difference was even more prominent after excluding patients with rectal cancer at baseline. Multivariate analysis confirmed that L-sided cancer was an independent factor for metachronous adenoma whilst advanced age, male gender, and longer follow-up time were other significant risk factors in this cohort.
There are several explanations for the higher risk of metachronous lesions in patients with L-sided cancer after surgical resection. Firstly, some of the adenomas identified on surveillance after resection of L-sided cancer could represent “missed lesion” not detected on prior clearing colonoscopy. It is well known that screening colonoscopy is less effective for the prevention of R-sided cancer, and interval cancers are more likely to be R-sided [22-25]. Study using back-to-back colonoscopies showed that R-sided adenomas were more often missed when compared to L-sided lesions [26]. Second, the rate of metachronous polyp or adenoma may be related to the biology of primary tumor. Recent data showed that CRC can be classified into 4 consensus molecular subtypes based on their molecular profile [27]. Some of the R-sided cancers are microsatellite unstable and their precursor lesions are serrated lesions. On the other hand, L-sided cancers are more likely associated with chromosomal instability and the precursor lesions can be adenomatous or serrated lesions [28]. Further studies to evaluate the molecular characteristics of primary tumor and metachronous polyps are warranted. Third, the incidence of R-sided cancer is increasing and is even more prevalent than L-sided cancer especially in the older age group [29-31]. A recent study also showed that the majority of adenomas were found in the proximal colon for patients beyond age 59 years [32,33]. While the mean age of our patients was 66.1 years, the “proximal shift” of colonic neoplasia may account for the higher risk of metachronous lesions found on surveillance colonoscopy in patients with L-sided tumor. The discrepant lengths of the remaining colon after R-sided or L-sided colectomy might also influence the adenoma recurrence rate.
It is interesting to note that the rate of metachronous polyp and adenoma in patients with rectal cancer was lower than in patients with carcinoma of sigmoid and descending colon. As the proportion of patients with fair to good bowel preparation on clearing colonoscopy and mean number of surveillance colonoscopy performed in both groups were comparable (rectal cancer: 86.2%, carcinoma of sigmoid and descending colon: 89.2%, P =0.51; rectal cancer: 1.48, carcinoma of sigmoid and descending colon: 1.52, P =0.71 respectively), the result cannot be explained by these factors. Recent study showed that there is difference in molecular profiles of colon cancer and rectal cancer [34] and the gut microbiome pattern may also change after treatment of colon tumour [35-37]. We postulate that these factors may contribute to the different rates of metachronous adenoma after surgical resection of tumor at different sites.
Our study showed that the rate of metachronous cancer was higher in patients with R-sided cancer when compared to patients with L-sided cancer. R-sided CRC may harbor more microsatellite unstable tumor, which was shown to be an independent risk factor for metachronous cancer [4,38]. Although this is in accordance with previous reports [9,11], the result has to be interpreted with caution as there were only 7 patients who developed metachronous cancer. Amongst the 5 R-sided cancer patients who had metachronous cancer, 1 patient had a second CRC detected at 11 months. The quality of bowel preparation of clearing colonoscopy was rated as poor in 1 patient and “not documented” in another patient. Second CRC in these patients may represent missed synchronous cancer. It is also noteworthy that for all R-sided CRC patients who developed metachronous cancer, there were no metachronous polyps detected. Similarly, precancerous lesions may be missed or resected incompletely on initial colonoscopy as a result of suboptimal bowel preparation, leading to metachronous cancer [39].
Current recommendation for patients with colon cancer is to perform colonoscopy 1 year after resection, and then every 3 to 5 years depending on the last colonoscopy findings. Patients with rectal cancer are recommended to have additional sigmoidoscopy as they have a higher rate of local recurrence [13-16]. As yet, there is no recommendation to stratify cancer patients into R-sided or L-sided. Intuitively, the results of our study may suggest a need to stratify surveillance colonoscopy after curative resection for R-sided or L-sided cancer. For example, young female patient with R-sided cancer may need less frequent surveillance colonoscopy after resection, while male patient with L-sided cancer may need more frequent surveillance colonoscopy. With this risk-stratified approach, a more efficient use of colonoscopy resources may be achieved.
Recently, there is increasing emphasis on the quality of colonoscopy, particularly on the adenoma detection rate. Several factors including bowel preparation, type of colonoscopes and even the endoscopists are all linked to adenoma detection rate during screening colonoscopy [40-43]. In this study, the bowel preparation was rated as good to fair in 89.7% and 90.4% of the clearing colonoscopies and surveillance colonoscopies respectively, which could be considered satisfactory. Although colonoscopies were all performed by experienced endoscopists or trainees under direct supervision, the colonoscope withdrawal time was not documented in this study as many colonoscopies were performed in the period before the benefits of slow withdrawal of colonoscope come into light [44,45].
Our study has other limitations. This is a retrospective study including all patients who had undergone curative colonic resection in our hospital during the defined study period. There were some differences in the baseline characteristics of the 2 groups of patients. In particular, the mean age of patients with R-sided cancer group was higher than patients with L-sided cancer (68.2 years vs. 65.1 years, P =0.02), which is not unexpected as R-sided cancer is associated with ageing. As age is also a risk factor for adenoma detection, the lower rate of metachronous polyp and adenoma in patients with R-sided tumor would support the difference found in this study was not due to difference in the mean age between the 2 groups. Second, we also included patients with rectal cancer in this study. Due to high local recurrence risk of rectal cancer, patients should undergo more regular sigmoidoscopic examination than those with more proximal cancers. Hence, there may be a chance of earlier detection of distal colonic neoplasm in these patients. Notably, we found that the rate of metachronous adenoma or polyp in patients with rectal cancer was comparable to those with R-sided cancer (Fig. 3). The difference between R-sided and L-sided cancer after excluding rectal cancer was even more significant (Table 3) and hence, our results could not be explained by the inclusion of patients with rectal cancer.
In conclusion, we found that the rate of metachronous adenoma after curative bowel resection was significantly higher in patients with prior L-sided than R-sided cancer. Lsided cancer, older age, male gender and longer follow-up time were all independently associated with metachronous adenoma detection after curative resection of CRC. Our data may support a need for the differential intervals of surveillance colonoscopy according to individual risk of metachronous adenoma, including the site of primary tumor.
Notes
FINANCIAL SUPPORT
The study was supported by the Li Shu Fun Medical Foundation and the Hui Hoy & Chow Sin Lan Charity Fund Limited to WKL.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION
YF Lam was involved in study concept and design, acquisition, analysis and interpretation of data and drafting of manuscript. WK Seto, KS Cheung, O Lo, I Hung and WL Law were involved in interpretation of data and critical revision of the manuscript. T Tong was involved in acquisition and interpretation of data. WK Leung was involved in study concept and design, analysis and interpretation of data, critical revision of manuscript and overall study supervision.