The Effect of Indigocarmine on Improvement of the Polyp Detection Rate during Colonoscopic Examination with Hood Cap
Article information
Abstract
Background/Aims
Hood cap-assisted chromocolonoscopy using indigocarmine is expected to improve the detection rate of colorectal polyps, especially adenomatous polyps. Therefore, aim of the present study was to evaluate the usefulness of hood cap-assisted chromocolonoscopy in routine colonoscopic examinations.
Methods
From January, 2013 through March, 2013, a total of 86 patients were enrolled (M:F=33:53, mean age=60 years). For each patient, hood cap-assisted colonoscopic examination was performed, followed by hood cap-assisted chromocolonoscopy using 0.2% indigocarmine from the cecum to the hepatic flexure. Total numbers and characteristics of polyps were compared before and after indigo carmine dye spraying.
Results
Prior to dye spraying, 48 polyps were found in 37 patients, and after dye spraying, 53 additional polyps were found in 34 patients. Of these undetected polyps, 45 (85%) were small sized polyps (≤0.5 cm). Histologically, 19 (36%) were adenomatous polyps, and of these, 15 (28%) were tubular adenomas and 4 (8%) were serrated adenomas. As for the polyp detection rate, there was no difference between the expert and the non-expert groups.
Conclusion
Hood cap-assisted chromocolonoscopic examination using indigocarmine was helpful in detecting cecum and ascending colon polyps, especially small sized polyps (<0.5 cm) and neoplastic polyps.
INTRODUCTION
Colon cancer is one of the most commonly diagnosed cancers in both men and women in the United States and Europe.1 As the incidence of colon cancer continues to increase, it has become the fourth leading cause of cancer-related deaths in Korea.2 For these reasons, performing colonoscopic exams is crucial for early diagnosis of colon cancer. Colonoscopy is one of the most effective procedures to detect and remove colorectal polyps, however, there are a number of polyps that are not detected clinically during screening colonoscopies.2 To improve polyp detection rates, hood cap-assisted colonoscopies and chromocolonoscopies using indigocarmine have been employed.
According to Brooker et al.3, no difference was found in the adenomatous polyp detection rate in all parts of the colon during chromocolonoscopy. However, the detection rate of small-sized adenomatous polyps was found to be significantly higher in the proximal colon. A domestic study also reported that chromoendoscopy using indigocarmine could improve the diagnostic rate of small adenomas and non-polypoid adenomas.4 Moreover, the use of indigocarmine chromoendoscopy was found to diagnose patients with ulcerative colitis more accurately in the colon.5 However, chromoendoscopy with indigocarmine has not yet been demonstrated to improve the detection rate of adenomatous polyps in the colon.6 In addition, the polyp detection rate was reported to be higher in hood cap-assisted colonoscopy as compared to standard colonoscopy.7
Therefore, the aim of the present study was to examine possible improvements in the detection rate of polyps and adenomas in the ascending colon by spraying indigocarmine during hood cap-assisted colonoscopy.
METHODS
1. Subjects
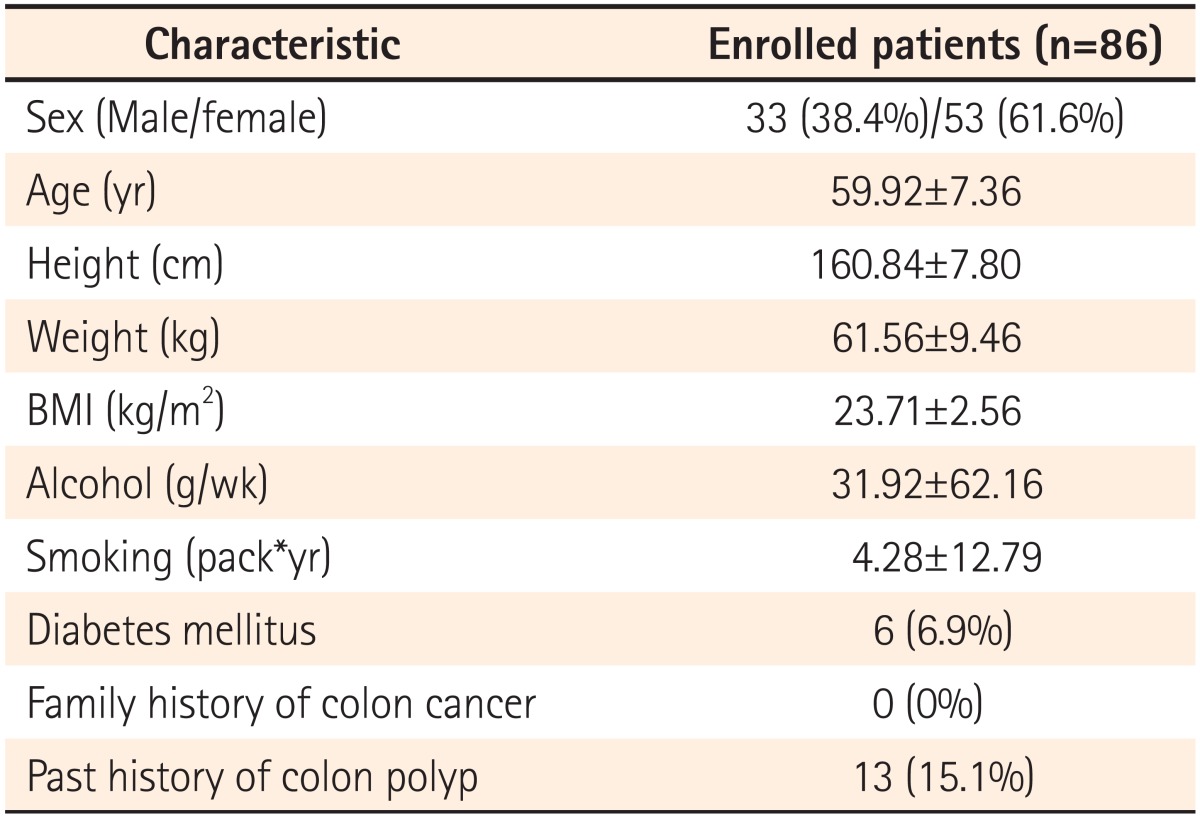
A prospective study was performed on 86 patients, all of whom consented to participate in the study, who underwent colonoscopy in the Department of Gastroenterology at Busan St. Mary's Hospital from January through March, 2013. Thirty-three of the study subjects were male and 53 were female, with a mean age of 60 years (range, 50-80 years). Investigators examined subjects' height, weight, BMI, tobacco and alcohol consumption, the presence of diabetes mellitus, a family history of colon cancer, and a history of colorectal polyps. Tobacco consumption was computed by multiplying the number of cigarettes smoked per day by smoking duration. Alcohol consumption was defined as the average amount of alcohol consumed per week in grams (g). The causes for performing colonoscopy were health screening in 44 subjects (51.2%), abdominal pain or distension in 24 subjects (27.9%), diarrhea in 8 subjects (9.3%), constipation in 8 subjects (9.3%), and bleeding in 2 subjects (2.3%). The study excluded subjects with polyposis syndrome, hereditary non-polypoid colorectal cancer, or inflammatory bowel disease, those with a history of colectomy or of emergency colonoscopic exams, as well as those with suspected colonic obstruction or who had been referred for polypectomy due to polyps detected in other hospitals. Subjects with poor bowel cleansing or incomplete colonoscopic exams, or those undergoing anticoagulant therapy or without consent forms for participation were also excluded.
2. Bowel Preparation
Overall bowel cleanliness was assessed and categorized based on the Aronchick scale,8 with excellent defined as a small volume of clear liquid or greater than 95% of the surface observable, good defined as a large volume of clear liquid covering 5% to 25% of the surface but greater than 90% of the surface observable, fair defined as detecting some semi-solid stool that could be suctioned or washed away but greater than 90% of the surface observable, and poor defined as detecting semi-solid stool that could not be suctioned or washed away and less than 90% of the surface observable. Inadequate preparation was defined when repreparation was needed due to a large amount of remaining stool.
3. Hood Cap Colonoscopy
Colonoscopies were conducted by four gastroenterologists who were divided into expert and non-expert groups of 2. Expert gastroenterologists had performed more than 1,000 colonoscopies over the course of more than 5 years, while the other group consisted of less experienced gastroenterologists. For colon cleansing, all subjects were administered 2-4 L of polyethylene glycol electrolyte solution 6-8 hours before colonoscopies. Unless contraindicated, colonoscopies were performed under a conscious sedation state through the administration of 25 mg of pethidine and 3-5 mg of midazolam. Colonoscopic exams were performed using a colonoscope (CF-H260AL, Olympus, Tokyo, Japan) with a hood cap (MH-466, Olympus, Tokyo, Japan) attached to the distal end.
A colonoscope with a hood cap attached was first inserted into the cecum and an examination was conducted from the cecum to the hepatic flexure. The location, size, and shape of detected polyps were recorded and biopsies were performed. After biopsies, indigocarmine dying was performed from the cecum to the hepatic flexure by spraying 20 mL of 0.2% indigocarmine using a spray catheter (PW-5V-1, Olympus, Tokyo, Japan). Colonoscopies were conducted again to re-examine the cecum, ascending colon, and hepatic flexure. The location, size, and shape of additionally detected polyps were also recorded. Additionally detected polyps were removed using biopsy forceps or through polypectomy using a loop snare. The histologic types of removed polyps were interpreted by a pathologist.
The study was performed after gaining the approval of the Busan St. Mary's Hospital Research Ethics Committee (BSM 2012-20).
4. Statistical Analysis
The study sample size was 77.0282 at a significance level of 5% and a power of 80%. By taking a withdrawal rate of 10% into consideration, a total of 86 subjects were included. All values were presented as mean±SD, sample number, or percentage. Paired t-tests were performed to compare the number, shape, size, and histologic type of polyps before and after indigocarmine dying. Independent-sample t-tests were conducted for the comparison between expert and non-expert groups. Differences were considered statistically significant at P<0.05. Statistical analysis was performed using PASW statistics 18.0 (IBM Co., Armonk, NY, USA) for Windows.
RESULTS
The present study involved 86 subjects (33 males and 53 females), with a mean age of 60 years (range, 50-80 years) (Table 1). The use of hood caps and indigocarmine did not result in any complications. The mean observation times from the cecum to the hepatic flexure before and after indigocamine dying were 228 seconds and 353 seconds, respectively. The observerd bowel cleanliness of the ascending colon was excellent in 4 subjects, good in 47 subjects, fair in 35 subjects, and there were no subjects with poor or inadequate preparation.
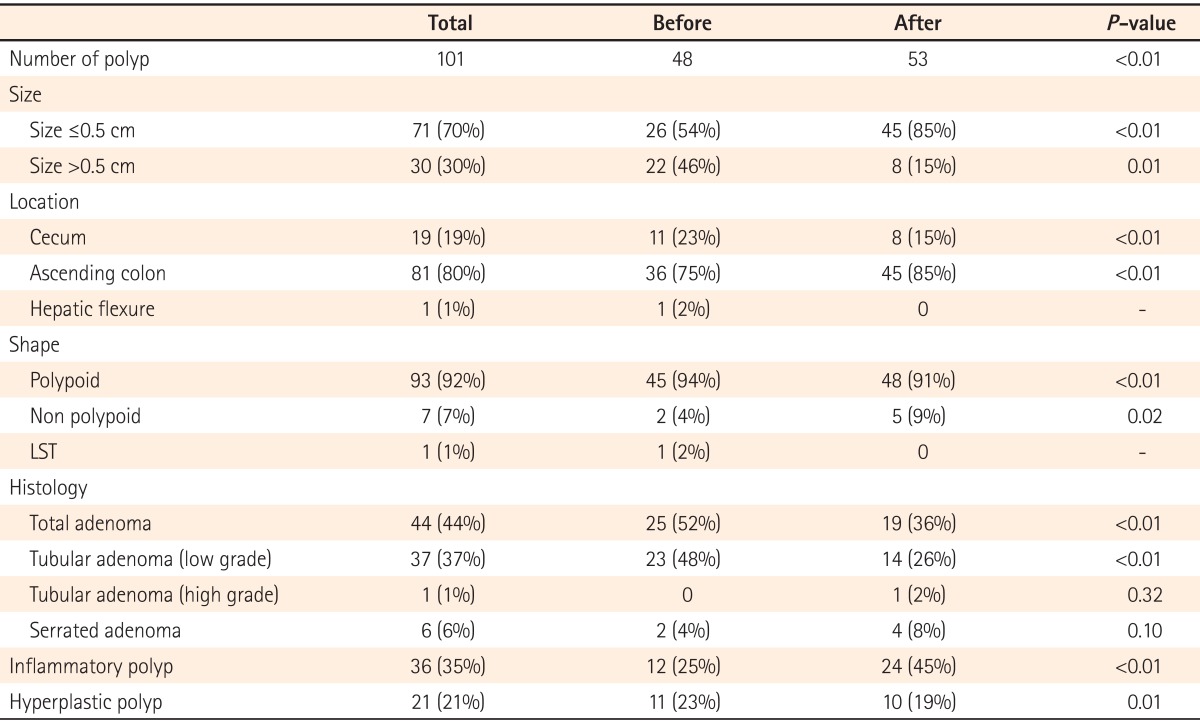
Before indigocarmine spraying, 48 polyps (mean 0.56) were found in 37 patients. After indigocarmine spraying, an additional 53 polyps (mean 0.62) were detected in 34 patients. Among those patients, polyps were newly discovered in 23 subjects who did not have any detected before dying. After staining, 101 polyps (mean 1.17) were detected in a total of 60 patients, which was approximately 2.1-fold higher than the number of polyps detected before dying (P<0.01).
Among the 48 polyps detected before dying, 26 (54%) were smaller than 0.5 cm and 22 (46%) were larger than 0.5 cm. Among the 53 polyps detected after staining, 45 (85%) were smaller than 0.5 cm and 6 (15%) were larger than 0.5 cm. The majority of the additional polyps detected after dye spraying were smaller than 0.5 cm (Table 2).
Regarding polyp locations, 11 (23%) were detected in the cecum, 36 (75%) in the ascending colon, and 1 (2%) in the hepatic flexure before dye spraying. After dye spraying, 8 (15%) polyps were detected in the cecum and 45 (85%) were detected in the ascending colon. No polyps were detected in the hepatic flexure after dye spraying. The polyp detection rate was slightly higher in the ascending colon after staining as compared to before staining.
Before dye spraying, 45 (94%) of the detected polyps were polypoid in shape, 2 (4%) were non-polypoid in shape, and 1 (2%) was a laterally spreading tumor. After dye spraying, 48 (90%) of the detected polyps were polypoid in shape, 5 (9%) were non-polypoid in shape, and none were laterally spreading tumors. The detection rate of non-polypoid polyps was significantly higher after indigocarmine spraying as compared to before spraying (P=0.02).
Results from the assessment of the histologic types of detected polyps revealed that 23 (47%) were tubular adenomas with low grade dysplasia, 2 (4%) were serrated adenomas, 12 (24%) were inflammatory polyps, and 11 (22%) were hyperplastic polyps before staining. After dying, 14 (26%) of the detected polyps were tubular adenomas with low grade dysplasia, 1 (2%) was a tubular adenoma with high-grade dysplasia, 4 (8%) were serrated adenomas, 24 (45%) were inflammatory polyps, and 10 (19%) were hyperplastic polyps. Neither villous adenoma nor carcinoma was detected before or after staining. Adenomatous polyps accounted for 19 (36%) of the additional 53 polyps detected after staining. A total of 44 adenoma polyps were found and this number was significantly higher than the 25 adenoma polyps detected before staining (P<0.01).
The difference in the polyp detection rate was compared between 2 groups as a function of the reasons for performing colonoscopies. The average numbers of detected polyps were 0.45 vs. 0.68 (P=0.16) before dying and 0.64 vs. 0.59 (P=0.80) after dying, respectively between the groups who underwent colonoscopies for regular check-up and the other group who underwent colonoscopies due to abdominal pain, diarrhea, constipation, bleeding, or other symptoms. The average total number of detected polyps between the 2 groups, 1.10 vs. 1.27 (P=0.48), showed no significant difference. Additionally, there were no significant differences in the detection rate of large polyps greater than 0.5 cm or adenomas between the 2 groups.
In the comparison of groups according to bowel cleanliness (excellent, good, and fair), average detection rates were 1.00, 1.13, and 1.29 polyps, respectively. The polyp detection rate was found to be higher in the fair group, however, no significant difference was observed. Furthermore, no significant differences were observed in the detection rates of polyps larger than 0.5 cm or adenomas.
When considering differences between the expert and non-expert groups, observation times were significantly shorter in the expert group (242 seconds) as compared to the non-expert group (338 seconds) before dye spraying (P=0.04). No significant differences between the 2 groups were observed in the number of polyps detected before and after indigocarmine dying (Table 3).
DISCUSSION
Colorectal polyps have profound clinical significance because they are highly correlated with the incidence of colon cancer. Colon cancers generally develop from benign adenomas. Hence, the removal of adenomatous polyps can reduce the risk of colon cancer incidence.9 Although colonoscopy is recognized as one of the most effective screening exams in detecting adenomatous polyps,4 even highly experienced gastroenterologists fail to detect 15%-27% of small adenomas.10,11 Recent investigations have revealed that non-polypoid polyps in particular are more likely to progress to cancer. However, the likelihood of missing non-polypoid polyps is higher in colonoscopy.4
Therefore, the present study assessed detection rates for all polyps and adenomatous polyps when chromoendoscopy was performed in addition to hood cap-assisted colonoscopy toward the goal of improving colon polyp and adenoma detection rates.
Hood cap-assisted colonoscopy increases visibility in parts of the colon by pressing the wrinkled colon surface using a transparent plastic cap that covers the distal end of the colonoscope. This technique should theoretically improve the detection rate of adenomas compare to standard colonoscopy. According to a meta-analysis conducted by Ng et al.,7 although hood cap-assisted colonoscopy did not increase the detection rate of adenomatous polyps in the colon, the overall detection rate of polyps was enhanced by 1.08-fold. In addition, the average insertion time of the colonoscope up to the cecum was reduced by 0.64 minutes. However, whether or not the use of the hood cap increases the detection rate of adenomatous polyps remains unknown.7
Chromocolonoscopy was first introduced in the 1970's and allows for a clearer view of the colonic mucosa and fine mucosal structures of lesions, enabling an enhanced adenoma detection rate.12 The use of indigocarmine is cost-effective and safe. Indigocarmine is a commonly used substance barely absorbed by the colon. Thus, it can be used up to 2.5 mg/kg.12
Controversy remains as to whether or not indigocarmine-assisted chromocolonoscopy increases the detection rate of adenomatous polyps.6 Mitooka et al.13 reported that the detection rate of colonic polyps was higher than those reported in previous studies that employed a capsule of indigocarmine solution administered 30 minutes before taking a polyethylene glycol electrolyte solution for bowel cleansing.13 Results from a study conducted by Kiesslich et al.1 also suggested that chromocolonoscopy with indigocarmine aided in the detection of small non-polypoid adenomatous polyps. According to results from studies conducted by Hurlstone et al.14 and Togashi et al.,6 indigocarmine-assisted chromocolonoscopy increased the detection rate of adenomas by 1.96 and 1.84-fold, respectively. Unfortunately, indigocarmine dye spraying stained the colonic muscosa blue and made detection of lesions such as angiodysplasia or colitis more difficult. For this reason, a thorough examination of the colon is recommended before dye spraying.1 Because the detection rate of adenomatous polyps was relatively lower in the rectum and sigmoid colon than in the proximal colon,3 performing chromocolonoscopy in the proximal colon with a higher detection rate of adenomas is thought to more suitable in determining its effectiveness.
In the present study, the ascending colon was examined using hood cap-assisted colonoscopies. Subsequently, the detection rate of polyps observed in the ascending colon after spraying indigocarmine was evaluated by re-performing hood cap-assisted colonoscopies. A total of 101 polyps were found in this manner and demonstrated a 2.1-fold increase in the polyp number detected before staining. Furthermore, 85% of newly detected polyps after staining were smaller than 0.5 cm and 36% were adenomatous polyps (15 tubular adenomas [28%] and 4 serrated adenomas [8%]). The adenoma detection rate was increased by 1.76-fold after staining. The results of the present study are in agreement with those of Brooker et al.3 who suggested that chromocolonoscopy is a safe and simple technique to improve the detection rate of small adenomas in the proximal colon. While it is clear that non-polypoid polyps are easily missed using standard colonoscopy, 5 of 7 non-polypoid polyps were detected after staining in the present study.
In several comparative studies on hood cap-assisted and standard colonoscopies, different results have been reported regarding the detection rate of colorectal polyps. In the meta-analyses conducted by Ng et al.7 and Westwood et al.,15 the mean number of polyps detected in hood cap-assisted colonoscopy was 0.41 in the colon, representing an increase in the detection rate of 1.08-fold compared to standard colonoscopy. According to the results of domestic studies conducted by Choi et al.16 and Choi et al.,17 the mean numbers of polyps detected from the cecum to the hepatic flexure were 0.33 and 0.32, respectively, during hood cap-assisted colonoscopy. Because the present study involved the performance of hood cap-assisted colonoscopies both before and after indigocarmine dye staining, a simple comparison with previous studies cannot be achieved. However, the mean polyp number detected in the present study was 0.57 in hood cap-assisted colonoscopies alone prior to staining and demonstrated a higher polyp detection rate than previous studies. After indigocarmine dye staining, the overall detection rates were 2.1 and 1.76-fold higher for polyps and adenomas, respectively. In the studies conducted by Brooker et al.,3 Hurlstone et al.,14 and Togashi et al.,6 the overall detection rates were 1.21, 1.44, and 2.23 polyps and 0.79, 0.88, and 1.13 adenomatous polyps, respectively, after indigocarmine dye staining. Although these values were higher than the overall detection rates of 1.19 polyps and 0.51 adenomatous polyps observed in the present study after staining, Brooker et al.3 investigated the entire colon including the cecum, ascending colon, transverse colon, and descending colon, and Hurlstone et al.14 and Togashi et al.6 observed polyps and adenomas in the entire colon. Because the present study examined the cecum and ascending colon only, comparisons with the results of the aforementioned previous studies may not be appropriate. Furthermore, ethnic differences in rates of polyp and adenoma occurrence are thought to be present between East Asians and Westerners and may add an additional layer of confound to comparisons between the results of these studies. Therefore, additional studies will be necessary to further investigate whether performing colonoscopy with indigocarmine dye spraying combined with hood cap-assisted colonoscopy improves the detection rate of polyps and adenomas as compared to using indigocarmine dye spraying alone.
The present study had several limitations. Because the study involved a relatively small sample size, only 1 laterally spreading tumor was observed before staining and was not additionally detected after staining. Thus, it was not possible to conclude whether indigocarmine dye staining increased the detection rate of laterally spreading tumors. Moreover, polyps should be evaluated at a standard of 0.5 cm, as there was only 1 polyp greater than 1 cm detected. Therefore, a larger-scale study comprising larger sample sizes would be necessary to determine whether indigocarmine staining can increase the detection rates of laterally spreading tumors and polyps larger than 1 cm. Additionally, examination time may have been extended due to indigocarmine staining. In the present study, indigocarmine spraying and suctioning time were included in the observation time measured after staining. Therefore, the observation time after staining was always longer than the observation time before staining. This challenge may be resolved by the development of more effective ways to spray indigocarmine.
Notes
Financial support: None.
Conflict of interest: None.