Intraoperative inspection alone is a reliable guide to the choice of surgical procedure for enteroenteric fistulas in Crohn's disease
Article information
Abstract
Background/Aims
Resection of the diseased segment and suture of the victim segment is recommended for enteroenteric fistula in Crohn's disease (CD). The main difficulty in this procedure remains reliable diagnosis of the victim segment, especially for fistulas found intraoperatively and inaccessible on endoscopic examination. We aimed to explore whether intraoperative inspection alone is reliable.
Methods
Patients undergoing conservative surgery between 2011 and 2016 for enteroenteric fistulas complicating CD were identified from a prospectively maintained database. Patients were divided according to whether the victim segment was evaluated by preoperative endoscopy + intraoperative inspection (PI group) or by intraoperative inspection alone (I group). Outcomes were compared.
Results
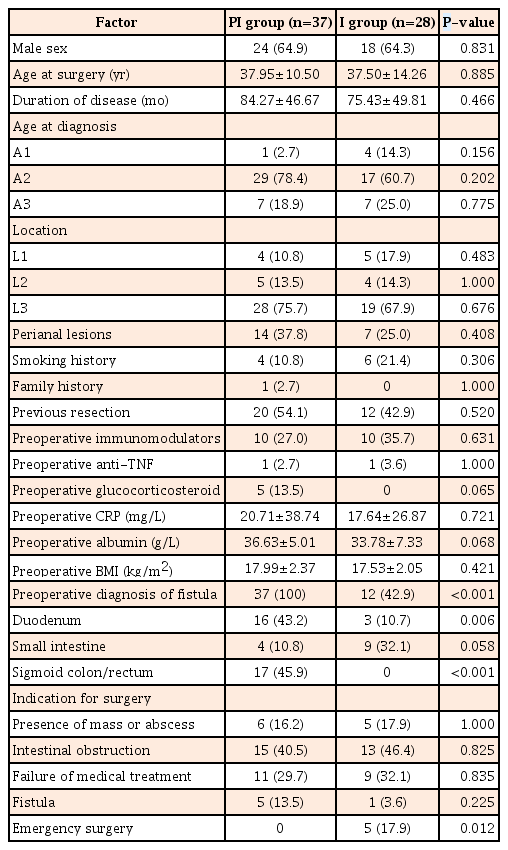
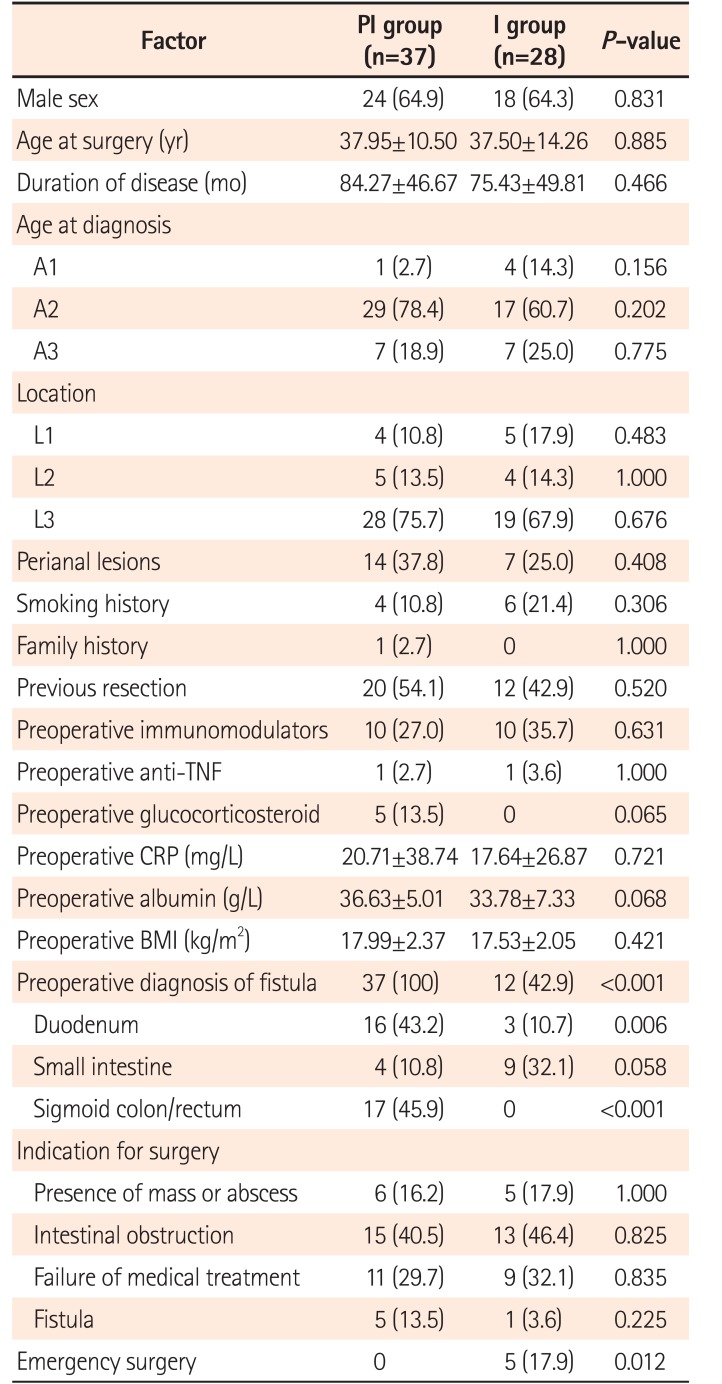
Of 65 patients eligible for the study, 37 were in in the PI group and 28 were in the I group. The baseline characteristics were similar between the groups, except for the rate of emergency surgery (0/37 in PI group vs. 5/28 in I group, P=0.012). Fistulas involved more small intestines (4/37 in PI group vs. 15/28 in I group, P<0.001) and fewer sigmoid colons (17/37 in PI group vs. 4/28 in I group, P=0.008) in I group due to accessibility with endoscopy. No difference was found in postoperative complications, stoma rates, postoperative recurrence, or disease at the repair site between the 2 groups (P>0.05).
Conclusions
For fistulas found intraoperatively and inaccessible on endoscopic examination, intraoperative inspection was a reliable guide when choosing between en bloc resection and a conservative procedure.
INTRODUCTION
Enteroenteric fistulas occur in 5% to 30% of patients with CD, and often require surgical treatment.12 Usually, enteroenteric fistulas in CD arise from a diseased segment of bowel and extend to a victim segment that is free from CD. As repeated resection is always required for recurrent disease in CD, in order to preserve as much intestine as possible, en bloc resection of fistulas (resection of both segments) is not recommended. Instead, a more conservative procedure (resection of diseased segment and suture of the victim segment) is advocated.3
Previous studies confirmed the conservative procedure was safe when suture repair was performed on the CD-free victim segment.45678 However, when the sutured segment was affected by CD, the risk of postoperative fistula or disease recurrence was increased.8 Therefore, the evaluation of the victim segment is essential for the decision on whether suture repair can be performed. Preoperative endoscopy with biopsies is the most accurate method for assessment of the victim segment, but it is only possible when the fistula is accessible, as in an ileosigmoid or coloduodenal location. However, more than 20% of enteroenteric fistulas are incidental intraoperative findings.7 For fistulas that are difficult to reach with endoscopy, intraoperative inspection (macroscopic observation and tactile assessment) remains the most feasible approach to the choice of surgical procedure. The present study aimed to determine whether intraoperative inspection alone is a reliable guide compared with preoperative endoscopy + intraoperative inspection when choosing between en bloc resection and a conservative procedure in CD complicated by an enteroenteric fistula.
METHODS
1. Patients
Approval from the Institutional Review Board of Jinling Hospital was obtained (2015NZKY-25), and the study was performed according to the principles of the Declaration of Helsinki. Eligible patients gave written informed consent. All patients undergoing surgical treatment at the IBD center in Jinling Hospital for enteroenteric fistulas complicating CD between January 2011 and August 2016 were identified from a prospectively maintained database. When additional data were required, medical records were reviewed. Only patients receiving suture closure of the defect on the victim segment were included in this retrospective study. The diagnosis of CD was endoscopically and histologically confirmed, and the diagnosis of enteroenteric fistula was confirmed by radiographic, endoscopic, and/or intraoperative findings. The following information was collected: sex, age at surgery, disease duration, disease phenotype according to the Montreal classification, smoking history, family history, preoperative medical treatment, and initial clinical manifestation. Details of surgical procedures, diameter of defects, postoperative complications, and CD recurrence were also obtained.
Preoperative total abdominal CT was routinely performed on all patients in our center, and preoperative colonoscopy was performed on those undergoing elective intestinal resection. Gastroduodenoscopy was performed when coloduodenal fistula was suspected. Fistula, abscess or phlegmon, and a star sign on CT, fistula and pseudopolyp on endoscopy, and fistula on fluoroscopic contrast studies were considered positive findings. When fistula was diagnosed or suspected, the intestine around the fistula was evaluated using endoscopy with biopsies if possible. Patients were divided according to whether the victim segment was evaluated by preoperative endoscopy + intraoperative inspection (PI group) or by intraoperative inspection (I group). In the PI group, suture repair of the victim segment was performed only when there was no evidence of disease on both preoperative endoscopy and intraoperative inspection. In the I group, whether suture repair was performed mainly depended on intraoperative findings (no obvious macroscopic disease such as stenosis, non-indurated tissue around the fistula, no ulcers on the mesenteric side, etc.).
2. Definitions
Postoperative complications were defined as any complication occurring within 30 days after surgery. Clinical recurrence was defined as a CDAI score >150 and CRP >10 mg/L. Endoscopic recurrence was defined as a Rutgeerts score of i2 or above.9 Surgical recurrence was defined as repeat resection for recurrent CD.
3. Surgical Technique
All operations were performed by the same team using open laparotomy. The judgement of whether the victim segment was CD-free was based on preoperative examination and intraoperative inspection in the PI group, and repair was performed only when the victim segment was confirmed normal by both preoperative endoscopy and intraoperative inspection in this group, and by intraoperative inspection alone in the I group. After the fistula was divided by finger fracture and sharp dissection, the diseased segment was resected. Stapled side-to-side anastomosis or temporary fecal diversion was carried out depending on the patient's intraoperative presentation and general condition. The defect on the victim intestine was trimmed until soft, and then closed using a double-layer submucosal continuous transverse repair with 4–0 Vicryl absorbable suture (Ethicon, Johnson and Johnson Inc., Somerset County, NJ, USA).10 For temporary fecal diversion, delayed anastomosis was performed 3 to 6 months later. For maintenance therapy, azathioprine (AZA) was given within 2 weeks after surgery; if not tolerated, infliximab or Tripterygium wilfordii Hook F was considered. All patients were followed by internet, telephone, or outpatient visits. Endoscopic follow-up was initiated 2 to 6 months after surgery, and then performed every 6 to 18 months. For these patients, the victim segment was assessed if accessible on endoscopic examination.
4. Statistics
All analyses were carried out using GraphPad Prism version 6 (GraphPad Software, San Diego, CA, USA). P-values <0.05 were considered significant. Categorical data were expressed as frequencies and percentages, and continuous data were expressed as mean±SD. The chi-square test was performed for group categorical data comparisons, and the t-test was used for group continuous data comparisons.
RESULTS
1. Baseline Characteristics
Of 65 patients (42 males and 23 females) who underwent intestinal repair during this period, 37 were in the PI group and 28 in the I group. The mean age at surgery was 37.75 years and the mean duration of disease was 80.46 months. The majority of patients belonged to A2 (46, 70.8%), and L3 (47, 72.3%) phenotypes according to the Montreal classification. Twenty-one (32.3%) had associated perianal disease, 10 (15.4%) were smokers, and only 1 had a family history. Previous resection had been performed in 49.2%. Nearly one-third received AZA preoperatively, while only 3.0% and 7.7% were treated with an anti-tumor necrosis factor (anti-TNF) agent or steroid. Abdominal pain was the most common initial clinical manifestation, occurring in 63.1%. About 75% (37/37 in PI group and 12/28 in I group, P<0.0001) of fistulas could be diagnosed before surgery using radiography or endoscopy. Intestinal obstruction and failure of medical treatment were the top 2 indications for surgery, while fistulas were the sole indication for surgery in only 6 patients (9.2%). Emergency surgery was performed in 5 patients (0 in PI group and 5 in I group, P=0.012). No other significant difference in baseline characteristics was found between PI and I groups (Table 1).
2. Diagnosis of Fistulas and Evaluation of Victim Segments
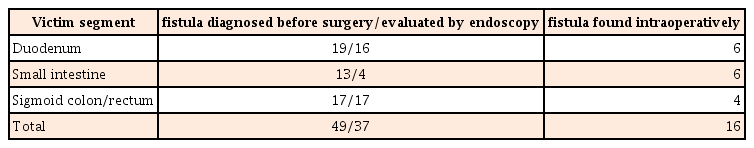
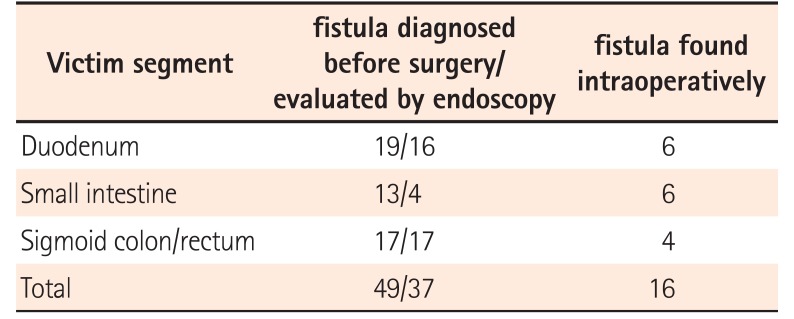
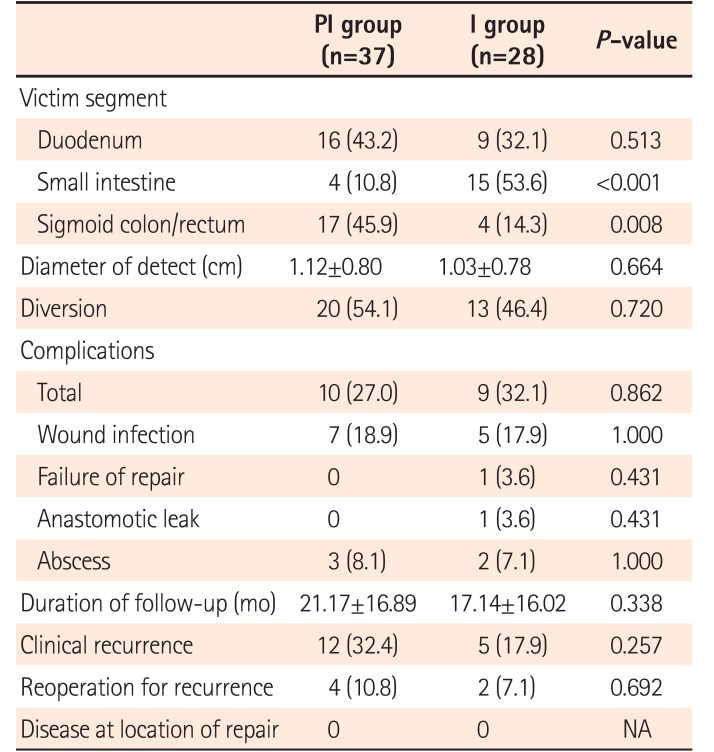
As all elective surgery patients (37 in PI group and 23 in I group) and 1 emergent surgery (perforation after endoscopy) patient in I group received preoperative colonoscopy, all sigmoid colon and rectum cases, except 4 requiring emergency surgery, were evaluated using endoscopy. Sixteen of 19 fistulas involving the duodenum could be diagnosed before surgery, and gastroduodenoscopy was performed in these patients. Due to difficult access, 3 fistulas could not be evaluated using endoscopy. Most fistulas between small intestinal segments could also be detected before surgery, but only 4 located on the terminal ileum could be reached and assessed using endoscopy (Table 2).
3. Short- and Long-Term Outcomes
The distribution of victim segments was different between PI and I groups. Due to inaccessible location on endoscopy, most small intestine fistulas were evaluated by intraoperative inspection. The size of the defect in the groups was similar. There was no death after surgery. The rate of temporary diversion was high in this cohort (54.1% in PI group and 46.4% in I group, P=0.720), but no permanent stoma was needed. Twelve cases (7 in PI group and 5 in I group, P=1.000) developed wound infections. One case in I group had anastomotic leakage, and 5 (3 in PI group and 2 in I group, P=1.000) had intra-abdominal abscess. Only 1 repaired defect (diameter, 4.0 cm) in the duodenum in I group failed (leakage at the site of repair). This patient was treated conservatively for 6 months, and then received successful suture repair. No difference in stoma rates or incidence of postoperative complications was found between the groups (Table 3).
The mean duration of follow-up was 21.17 months in PI group versus 17.14 months in I group (P=0.338). All patients received at least 1 endoscopic follow-up in this period. Overall, the rates of clinical, endoscopic, and surgical recurrence were 26.2%, 46.2%, and 9.2% respectively. Among the 65 patients who underwent endoscopy, information about 3 repairs in PI group (2 in the duodenum and 1 in the small intestine) was not collected because of inaccessible location on endoscopy. Of these 3 patients, 2 were free from CD clinical and surgical recurrence and had no evidence of anastomotic recurrence. The other patient underwent reoperation due to anastomotic stenosis. No disease around the location of repair was observed in either group. No difference in long-term outcomes was found between the groups (Table 3).
4. Risk Factors for Postoperative Complications
To determine whether the location of the victim segment was a confounding factor for postoperative complications and identify other risk factors for complications, univariate analysis was carried out. No factor was found to be associated with postoperative complications: repair at duodenum (P=0.579), repair at small intestine (P=1.000), repair at sigmoid colon/rectum (P=0.757), age at surgery (P=0.544), duration of disease (P=0.084), perianal lesions (P=1.000), smoking history (P=0.685), previous resection (P=0.558), preoperative immunomodulators (P=0.762), preoperative anti-TNF agent (P=1.000), preoperative glucocorticosteroid (P=1.000), preoperative CRP (P=0.600), preoperative albumin (P=0.071), preoperative diagnosis with fistula (P=0.170), and emergency surgery (P=0.076). Multivariate analysis was not performed because no risk factor was identified in univariate analysis.
DISCUSSION
Enteroenteric fistula is a common and intractable complication of CD, and surgery is still the mainstay of treatment. The complexity of this type of fistula results from more extensive intestinal inflammation, associated abdominal mass, conjunction with the abdominal wall, or enterocutaneous fistula. As surgery is not curative for CD, 24% of patients need reoperation within 5 years.11 Surgical treatment with en bloc resection of fistulas may lead to the loss of a large quantity of normal intestine and will increase the risk of intestinal failure. As an internal fistula in CD is often a communication between diseased intestine and a victim intestinal segment, a more conservative procedure, with resection of only the diseased intestine and preservation of the victim segment, has been recommended for decades.1213 Studies have confirmed this procedure is safe in CD ileosigmoid fistulas.
The main difficulty in the conservative procedure remains reliable diagnosis of the victim segment, especially in those fistulas found incidentally at the time of operation. If the victim segment affected by CD is left in place, an immediate (postoperative fistula) or late (recurrent disease) complication can develop. Endoscopy is the most useful examination for CD detection, and should be conducted if possible. CT can provide useful information for fistulas that are hard to reach on endoscopic examination and those found intraoperatively, but is not definitive. In the present study, we reviewed our experience of intestinal repair in fistulizing CD. Sixty-five repairs (25 in duodenum, 19 in small intestine, and 21 in sigmoid colon/rectum) were performed during a 5-year period. Results indicated that intraoperative inspection (macroscopic examination and tactile assessment) alone was a reliable guide when choosing between en bloc resection and a conservative procedure, and did not increase the risk of postoperative leak at the site of repair or postoperative disease recurrence.
Preoperative diagnosis of a fistula is important, and can help in the choice of appropriate examinations for the victim segment. Clinical symptoms and diagnostic methods such as endoscopy, CT, and barium enema are useful for the detection of internal fistulas.1415 More than 70% of fistulas can be detected before surgery. Other than direct evidence for internal fistulas on diagnostic examination, studies have reported that a “star sign” on MRI or CT, and segmental sigmoid polyposis can also be used as diagnostic markers of enteroenteric fistulas.1516
The value of preoperative endoscopic assessment of victim intestinal segments has been investigated in one study. Saint-Marc et al.8 compared 15 CD ileosigmoid fistula cases that underwent preoperative colonoscopy with 15 that did not, and found macroscopic intraoperative examination alone led to a 33% rate of incorrect evaluation of colonic involvement by CD. There was greater morbidity without preoperative colonoscopy, but the difference was not significant. This result suggested that microscopic disease may not affect outcomes in the conservative procedure. In the present study, 28 fistulas were found intraoperatively or could not be reached on endoscopic examination. The assessment of the presence of CD in victim segments mainly depended on macroscopic observation and tactile assessment, and both short- and long-term outcomes of these repairs were also satisfactory. Preoperative endoscopic assessment should be done if possible, but a decision based on intraoperative inspection alone is acceptable for fistulas found incidentally during surgery and inaccessible with endoscopy.
Although the majority of patients received preoperative optimization, the rate of temporary fecal diversion in this study was 50.8%, which was relatively high but consistent with previous reports (7.7%–51.0%).717 Possible explanations are as follows. First, patients treated in our highly specialized inflammatory bowel disease center may be more complex. This was reflected in the presence of multiple fistulas, more intra-abdominal abscesses, and poor general conditions in this study cohort. Second, in order to reduce the risk of repair failure, surgeons intuitively preferred to perform a protective stoma. The incidence of postoperative complications was 29.2%, and the incidence of intra-abdominal septic complications was 10.7%. These results are superior to those reported in a meta-analysis (20.2%) of open surgery in CD,18 but slightly higher than in a study that used similar preoperative management.17 Our results for clinical, endoscopic, and surgical recurrence are also comparable to those reported in a previous study.19 Postoperative AZA maintenance therapy, smoking cessation, and scheduled follow-up may contribute to the relatively low rate of recurrence.
This study has several limitations. First, because of the retrospective nature and small sample size, the results may be overestimated. Furthermore, this is a single-center study, and the surgical experience and practices may affect the results.
In conclusions, Enteroenteric fistula is a common complication of CD. When surgery is required, a conservative procedure (resection of diseased segment and suture of the victim segment) is recommended, and the preoperative evaluation of the victim segment is important for the choice of surgical procedure. For fistulas found intraoperatively and inaccessible on endoscopic examination (preoperative endoscopic evaluation of the victim segment was not possible), intraoperative inspection (macroscopic examination and tactile assessment) alone was a reliable guide when choosing between en bloc resection and a conservative procedure, and did not increase the risk of postoperative leak at the site of repair or postoperative disease recurrence.
Notes
FINANCIAL SUPPORT: The authors received no financial support for the research, authorship, and/or publication of this article.
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION: W.Z. conceived and designed the study. Z.G. and X.C. collected and interpreted data and wrote the manuscript. R.L. collected data. J.G., Y.L., and L.C. analyzed the dataset.