Korean Guidelines for Postpolypectomy Colonoscopic Surveillance: 2022 revised edition
Article information
Abstract
Colonoscopic polypectomy is effective in decreasing the incidence and mortality of colorectal cancer (CRC). Premalignant polyps discovered during colonoscopy are associated with the risk of metachronous advanced neoplasia. Postpolypectomy surveillance is the most important method for managing advanced metachronous neoplasia. A more efficient and evidence-based guideline for postpolypectomy surveillance is required because of the limited medical resources and concerns regarding colonoscopy complications. In these consensus guidelines, an analytic approach was used to address all reliable evidence to interpret the predictors of CRC or advanced neoplasia during surveillance colonoscopy. The key recommendations state that the high-risk findings for metachronous CRC following polypectomy are as follows: adenoma ≥10 mm in size; 3 to 5 (or more) adenomas; tubulovillous or villous adenoma; adenoma containing high-grade dysplasia; traditional serrated adenoma; sessile serrated lesion containing any grade of dysplasia; serrated polyp of at least 10 mm in size; and 3 to 5 (or more) sessile serrated lesions. More studies are needed to fully comprehend the patients who are most likely to benefit from surveillance colonoscopy and the ideal surveillance interval to prevent metachronous CRC.
INTRODUCTION
Colonoscopy is currently a key diagnostic modality for colorectal cancer (CRC) screening and establishing a treatment strategy. CRC remains one of the leading causes of cancer-related deaths worldwide, despite a decreasing trend in its incidence and mortality owing to the development of screening methods and prevention programs [1]. Screening methods to prevent CRC have been presented in various national cohort studies. Among various methods, colonoscopic polypectomy, which involves the removal of colorectal polyps using colonoscopy, is reportedly the most effective method for reducing CRC incidence and CRC-related mortality [2,3]. Patients with colorectal polyps are at a high risk of developing colorectal polyps and CRC in the future; thus, appropriate surveillance using colonoscopy after colorectal polyp resection is instrumental [4,5]. Additionally, the significance of colonoscopic surveillance lies not only in detecting metachronous polyps but also in the additional detection of colorectal lesions not detected by index colonoscopy.
In South Korea, fecal occult blood tests have been adopted as a national test to screen for CRC. However, colonoscopy has already been considered for CRC screening because of the characteristics of the healthcare environment in Korea, defined by its high accessibility and utility of health services, leading to a remarkable increase in the diagnosis and resection of colorectal polyps [6]. Although there is no doubt regarding the importance of postpolypectomy colonoscopic surveillance, the method may have a marginal effect on prevention compared to a screening colonoscopy, and the likelihood of complications from colonoscopy is also present. Therefore, guidelines for the optimal practice of postpolypectomy colonoscopic surveillance, which maximize the benefits and minimize the possible damage, are required [2,7,8]. To establish Korea-specific guidelines, the Korean Society of Gastroenterology, the Korean Society of Gastrointestinal Endoscopy, the Korean Association for the Study of Intestinal Diseases, and the Korean Society of Abdominal Radiology jointly organized a multi-society Taskforce Committee to develop national guidelines for colorectal polyp treatment. The Korean Guidelines for Postpolypectomy Colonoscopic Surveillance were first published in 2012 and were distributed to health professionals for use in clinical practice [9]. Since establishing the first Korean guidelines, many studies have reported postpolypectomy colonoscopic surveillance, necessitating a revision of the existing Korean guidelines to reflect and incorporate additional evidence and reports. The revised edition of the Korean Guidelines for Postpolypectomy Colonoscopic Surveillance was developed by adapting 3 international guidelines that have been recently revised and released [10-12]. In this way, we aimed to present revised, evidence-based guidelines, which can be used as a useful reference to determine the timing and interval of colonoscopic surveillance based on the assumption that the patient underwent a high-quality index colonoscopy conducted by a specialist in treating colorectal polyps. For major recommendations in the revised guidelines, CRC incidence and mortality were set as the primary endpoints. The risk of developing metachronous advanced neoplasia, which was set as a key endpoint in the previous guidelines, was considered a secondary endpoint. Estimates of benefits and risks were comprehensively considered in the revised guidelines. However, the revised guidelines exclude recommendations for follow-up of hereditary CRC (e.g., hereditary non-polyposis CRC and familial adenomatous polyposis), inflammatory bowel disease, and serrated polyposis syndrome. Additionally, these guidelines do not take precedence over clinical evaluations made by physicians taking into consideration various factors related to the patients and healthcare environment in real-world clinical practice. Nevertheless, these guidelines are expected to serve as useful and complementary references in clinical settings.
PROCESS OF GUIDELINE DEVELOPMENT
The guidelines are applied to all patients (both men and women, including those with comorbidities) who have undergone colonoscopy and have had polyps removed. The Guidelines Development Committee and Taskforce Committee include gastroenterologists and methodological experts as members to develop a revised edition of existing guidelines (Supplementary Material 1). By selecting reference guidelines through a systematic literature review and meta-analysis using a systematic process, the final guidelines were adapted for the development of this guideline. The guidelines developed in this study will be revised within the next 5 years, although early revision may be necessary in case of significant changes in the evidence base for the condition.
1. Development Procedure
The guidelines were developed based on the Guidance for the Development of Clinical Practice guidelines ver. 1.0, as published by the National Evidence-based Healthcare Collaborating Agency (Supplementary Material 2). The Guidelines Development Committee held its first meeting on July 6, 2020, and discussed the direction of revision for the existing guidelines.
The guideline development process consists of 3 stages: planning, development, and finalization. The planning stage consisted of (1) selecting the key themes of the guideline, (2) reviewing the existing guidelines, (3) establishing a development plan, and (4) selecting key questions. The development stage consisted of (5) searching for evidence, quality assessment, and synthesis; (6) writing recommendations, determining the strength of recommendation; and (7) drawing consensus. The finalization stage consisted of an external review and publication of the final guidelines.
1) Selection of Key Questions
The Task Force Committee, consisting of 9 members, reviewed 3 guidelines developed in the US (US Multi-Society Task Force [USMSTF]), Europe (European Society of Gastrointestinal Endoscopy [ESGE]), and the UK (British Society of Gastroenterology [BSG]). First, 12 related themes were selected. Detailed key questions were determined while taking into consideration the patient population (P), intervention (I), comparator (C), and outcome (O). Thus, the key questions that represent the building blocks of the recommendations are presented as PICO questions (Supplementary Material 2).
2) Search and Selection of Guidelines
The search for related literature was conducted by 2 taskforce members, using keyword terms for the guidelines. The major sources used for literature search included the international search engines PubMed, OVID-EMBASE, and Cochrane libraries. A total of 503 guidelines published after 2015 were retrieved after excluding duplicates. After reviewing the titles and abstracts, 55 articles were selected. By reviewing the original texts of the articles, 3 guidelines that satisfied the following 3 conditions were finally selected: (1) guidelines including PICO that matched the key questions; (2) evidence-based guidelines that included the report of a systematic literature search and showed a clear connection between the recommendations and the supporting evidence; and (3) guidelines published in English (Supplementary Material 3 and 4).
3) Final Selection Process of the Guideline
Through a systematic literature review and inclusion/exclusion criteria, a quality assessment was conducted for the 3 guidelines published by the USMSTF, ESGE, and BSG. All of them were selected as guidelines for adaptation (Supplementary Material 5 and 6) [10-12]. Quality assessment of the guidelines was performed based on the Korean Appraisal of Guidelines for Research and Evaluation II (K-AGREE II). In addition to comprehensive evaluation, scope and purpose, the rigor of development, stakeholder involvement, clarity of presentation, applicability, and editorial independence were considered the key assessment domains [13]. Quality assessment of the guidelines using the K-AGREE II was performed by 3 taskforce members per guideline, and items with a difference of more than a specified score among taskforce members were refined through re-review and consensus discussion. For the final selection of the guidelines, the rigor of development was considered with particular attention.
4) Writing Process of the Guideline
The recommendations and related evidence of the 3 guidelines selected by the Task Force Committee were comprehensively reviewed to derive the primary recommendations for key questions (Supplementary Material 7), and the acceptance and applicability of these recommendations were evaluated (Supplementary Material 8 and 9). Subsequently, the opinions of all the members were collected, and the final recommendations were compiled. For the level of evidence for each key question, major foreign grading methodologies, such as the Scottish Intercollegiate Guidelines Network, Grading of Recommendations, Assessment, Development and Evaluation, and existing domestic clinical practice guidelines, were reviewed [14-17]. After discussing with the Guidelines Development Committee, the level of evidence was divided into 4 levels, as shown in Table 1. To consider the level of evidence, the study design and quality assessment results of the selected literature were evaluated, and the consistency of the outcomes and precision of the evidence (total number of subjects or confidence intervals [CIs] in the included articles) were considered to determine the level of evidence for each key question. The strength of the recommendation was divided into 4 levels: strong recommendation, conditional recommendation, not recommended, and inconclusive (Table 2). For the content that lacked evidence or required clinical interpretation, the task force members held a consensus discussion to reach an agreed conclusion. The level of evidence was divided into 5 levels, and the strength of recommendation was determined by considering the level of evidence, benefits (such as clinical effects, increased patient satisfaction, and quality of life), and harm (such as adverse events, increased use of unnecessary resources, and decreased patient satisfaction). For cases where quantitative synthesis of evidence was judged to be possible, a meta-analysis was performed, and the effect of a specific intervention on the outcome was presented using relative risk (RR), each with a 95% CI. Rex version 3.5.0.2 (RexSoft Inc., Seoul, Korea; http://rexsoft.org/) was used for meta-analysis, with the “meta” R package:
Here, the I2 value ranged between 0 and 100%. An I2 value <25% indicated large homogeneity, an I2 of 25% to 50% indicated low heterogeneity, an I2 of 50% to 70% indicated medium heterogeneity, and an I2 ≥70% indicated high heterogeneity. The final strength of recommendation was determined by consensus of ≥80% of the members in principle but was ultimately determined based on the consent of all members of the Taskforce Committee.
5) Consensus and Adoption of Recommendations
After drafting the statements, the drafts were sent to experts in the relevant field by e-mail for review in advance, and the necessary modifications were made through teleconferencing. A modified Delphi technique was used for the revised statements to draw a consensus among multi-society and multi-institutional experts for final confirmation (Supplementary Material 2).
2. Terms and Definitions
In this guideline, based on the Korean Guidelines for Postpolypectomy Colonoscopic Surveillance published in 2012, the most commonly used terms were defined as follows, after referring to domestic and foreign studies [9].
(1) Postpolypectomy surveillance: Colonoscopy examination to detect synchronous and metachronous polyps for removal before becoming malignant after polypectomy. This term excludes the use of colonoscopy or other examinations to monitor recurrence after CRC treatment.
(2) Advanced adenoma: Adenomas ≥10 mm in size, with high-grade dysplasia/tubulovillous or villous adenoma.
(3) Advanced neoplasia: Advanced adenoma or CRC.
(4) Serrated polyp: The umbrella term used to describe hyperplastic polyps, sessile serrated lesions (SSLs), and traditional serrated adenomas (TSA) based on the pathological diagnostic criteria.
(5) Index colonoscopy: Colonoscopy was performed most recently before the surveillance colonoscopy. Index colonoscopy refers to a high-quality examination performed with adequate bowel preparation by colonoscopists who have received supervised endoscopy training above a certain level.
(6) Adequate bowel preparation: There is no consensus regarding the definition of adequate bowel preparation. ESGE defines adequate bowel preparation as follows: Boston Bowel Preparation Scale ≥6, Ottawa Scale ≤7, or Aronchick Scale excellent, good, or fair [11,18].
(7) High-quality examination: High-quality examination was defined based on various domestic and international guidelines and studies. Colonoscopy should be performed by a colonoscopist with an adequate adenoma detection rate (>30% for men, >20% for women). Patients undergoing colonoscopy should also undergo adequate bowel preparation. Colonoscopy should be performed up to the cecum, and the appropriate location (entire cecum, ileocecal valve, and appendiceal orifice) should be determined. The examination was completed after observing the colonic mucosa during sufficient withdrawal time [10,19,20].
(8) Index adenoma: An adenoma that serves as the most fundamental reference for colonoscopic surveillance. Among the adenomas found at index colonoscopy, the adenoma with the most advanced pathological findings is set as the index adenoma; however, if the pathological findings are the same, the index adenoma refers to the largest adenoma.
3. Limitations of the Revised Edition of the Korean Guidelines for Postpolypectomy Colonoscopic Surveillance
Most studies used as evidence in this guideline were performed in Western countries, and the number of studies with the Korean population was limited. Additionally, most foreign studies used evidence from observational studies rather than randomized controlled trials (RCTs), which limited the quality of evidence for this guideline. Therefore, the task force undertook a Delphi meeting with clinical experts to treat colorectal polyps to reflect the clinical setting in South Korea and explore metrics for treating colorectal polyps in clinical practice.
4. External Review
An external review was conducted with experts from the Korean Society of Coloproctology who did not directly participate in developing this guideline to objectively verify the prepared draft. The outcomes of the external review can be found in the Supplementary Material 10. After the review, the final statements were prepared and compiled by the Taskforce Committee, documented after the final review by the Guidelines Development Committee, and the final version of the guidelines was confirmed through a final review by the committee.
5. Distribution and Implementation of the Guidelines
The present guidelines will be published on the websites of relevant societies for viewing. Additionally, this guideline summarized the latest trends and global evidence on postpolypectomy colonoscopic surveillance through a systematic literature review and was published in the journals of relevant societies. Guidelines can also be used on various social media channels. To promote the implementation of the guidelines, a presentation in conference sessions is being considered, and changes in treatment patterns after releasing this guideline will be monitored. We aimed to monitor specific changes in treatment volume through open data sources, such as the Healthcare Bigdata Hub (https://opendata.hira.or.kr) or the Korea National Cancer Incidence Database.
6. Conflicts of Interest
All members who participated in developing the guidelines disclosed their real and explicit interests related to the development activities of the guidelines. None of the members, including the chairperson of the Guidelines Development Committee, had experience in the development or approval process of guidelines under review before the development of the present guidelines, nor had they had any relationship with companies related to medicines, commodities, and services related to the guidelines within the 2 years before developing the guidelines. Those who received research grants did not participate in the discussion or voting process when the medications of the applicable company were discussed. The authors received no financial support from institutions or organizations other than the Korean Society of Gastrointestinal Endoscopy, the Korean Society of Gastroenterology, and the Korean Association for the Study of Intestinal Diseases.
SUMMARY OF RECOMMENDATIONS
The guidelines assessed several risk factors to be reflected in determining the postpolypectomy surveillance interval and presented an appropriate surveillance interval based on the identified risk factors. The details of this summary are presented in Tables 3 and 4.
KEY QUESTIONS AND RECOMMENDATIONS
1. What Are the Risk Factors Related to CRC Incidence?
1) Is the Size of Tubular Adenoma a Risk Factor to be Considered When Shortening the Colonoscopic Surveillance Interval?
Statement 1. Shortening of the colonoscopic surveillance interval should be considered in patients with tubular adenomas ≥10 mm at index colonoscopy. (Strength of recommendation: Conditional recommenda-tion, Level of evidence: Low)
Similar to the 2012 domestic guidelines, a comprehensive review of studies reported since 2012 suggested that a large adenoma detected at index colonoscopy increased the future risk of advanced neoplasia development [9]. However, mixed results were reported depending on the cutoff size of the adenoma (10 mm vs. 20 mm). Overall, studies have shown consistency in that the index colonoscopy findings of adenomas >20 mm were associated with an increased risk of advanced neoplasia. However, with reference to the adenoma size (10 mm), there were disparities in the reported results. In a retrospective multicenter cohort study conducted in the UK in 2017 that included 11,944 patients with intermediate-risk adenomas at index colonoscopy, surveillance colonoscopy was reported to reduce the risk of CRC incidence in the group with 1 to 2 adenomas ≥10 mm in size or 3 to 4 adenomas <10 mm in size. The protective effect of surveillance colonoscopy was pronounced in patients with incomplete colonoscopy, poor bowel preparation, adenomas with high-grade dysplasia or large adenomas (≥20 mm), and proximal polyps at index colonoscopy [21]. However, in patients without these findings at the index colonoscopy, the protective effect of surveillance colonoscopy was not significant. In a study that analyzed the Polish national CRC screening program, patients with adenomas ≥20 mm in size (standardized incidence ratio [SIR], 2.07; 95% CI, 1.40–2.93) or with high-grade dysplasia (SIR, 0.79; 95% CI, 0.39–1.41) showed a significant increase in the risk of CRC [22]. A study conducted in the UK in 2020 reported no significant increase in future CRC incidence for adenomas with a size of 10–19 mm compared to those with a size of <10 mm (hazard ratio [HR], 1.30; 95% CI, 0.75–2.26) [23]. However, in a recent cohort study, the risk of future CRC increased in both cases of tubular adenoma (HR, 2.54; 95% CI, 1.39–4.64) and serrated polyps (HR, 2.82; 95% CI, 1.16–6.82) when the size was ≥10 mm [24]. In a case-control study conducted in the US, adenomas ≥10 mm in size were significantly correlated with an increase in CRC incidence within 10 years of the examination (odds ratio [OR], 2.38; 95% CI, 1.53–3.70) [25]. In summary, the risk of future advanced neoplasia increases when a large adenoma is detected, and the risk increases with an increase in the size of adenoma at index colonoscopy. Since most studies considered 10 mm as the reference value for adenoma size that indicates an increased risk of future CRC, and considering the recent reports that the future advanced neoplasia increased in adenomas with a size of 6 to 9 mm compared to those with a size of 1 to 5 mm [26,27], we used 10 mm as the cutoff value in this guideline, instead of 20 mm.
2) Is the Number of Colorectal Adenomas a Risk Factor That Should Be Considered When Shortening the Colonoscopic Surveillance Interval?
Statement 2. Patients with 3 to 5 non-advanced adenomas (NAAs) removed at index colonoscopy have the probability of developing metachronous CRC and the risk of metachronous advanced neoplasia; therefore, shortening the surveillance interval should be considered. (Strength of recommendation: Conditional recommendation, Level of evidence: Moderate)
Based on the results of recent large cohort studies, Western guidelines state that 3 to 4 NAAs do not increase the risk of metachronous advanced neoplasia, as with the 1 to 2 NAAs detected at index colonoscopy [11,12,21,22,28]. Although the BSG guidelines define ≥5 NAAs as high-risk findings related to metachronous CRC [12], the USMSTF guidelines still consider ≥3 NAAs as high-risk adenomas, citing insufficient research evidence on the risk of metachronous advanced neoplasia in patients with 3 to 4 NAAs [10].
Therefore, a meta-analysis was performed on previous cohort studies that evaluated the risk of metachronous advanced neoplasia and CRC between groups with the removal of 3 or ≥5 NAAs and those with the removal of 1 to 2 NAAs, without other high-risk findings at index colonoscopy. Among the studies included, there were differences in terms of patient eligibility criteria, the number of surveillance attempts, and the duration of follow-up. It was also difficult to determine the status of colonoscopy quality or use of high-definition endoscopy and the timing of surveillance. These studies also had different primary endpoints, making consistent comparison and analysis challenging [21,26,29-38].
Although there was statistical heterogeneity in the included studies, a meta-analysis of studies that included patients with 1 to 2 NAAs (n=27,638), ≥3 NAAs (n=4,973), and ≥5 NAAs (n=991) at index colonoscopy showed that during a mean follow-up duration of 4.9 years, the advanced neoplasia incidence rates were 5.4%, 10.3%, and 10.0%, respectively (Supplementary Fig. 1A). The RR of metachronous advanced neoplasia was 2.0 (95% CI, 1.78–2.26; P<0.001; I2=0%) and 2.2 (95% CI, 1.35–3.73; P=0.002; I2=62.2%), respectively, in the ≥3 NAAs or ≥5 NAAs group compared to the 1 to 2 NAAs group, indicating a significant increase in the risk of metachronous advanced neoplasia (Supplementary Fig. 1B). Although there was a limitation in that only 3 retrospective cohort studies were included in the analysis, the RR of metachronous advanced neoplasia was 1.26 (95% CI, 1.05–1.52; P=0.012; I2=0%) in the 3 to 4 NAAs group compared to the 1 to 2 NAAs group, indicating a statistical significance. In the ≥5 NAAs group compared to the 3 to 4 NAAs groups, the RR of metachronous advanced neoplasia was 1.96 (95% CI, 0.97–3.96; P=0.060; I2= 68.0%), suggesting a statistical tendency (Supplementary Table 1).
When a meta-analysis was conducted with the incidence and RR of metachronous CRC, the incidence of CRC was 0.2%, 0.5%, and 0.1% in the 1 to 2 NAAs, ≥3 NAAs, and ≥5 NAAs groups, respectively (Supplementary Fig. 2A). The RR of metachronous CRC was 1.80 (95% CI, 0.94–3.45; P=0.077; I2=0%) and 2.36 (95% CI, 0.22–25.94; P=0.481), respectively, in the ≥3 NAAs and ≥5 NAAs groups compared to the 1 to 2 NAAs group. Thus, the RR of metachronous CRC increased with an increase in adenomas; however, the difference was not statistically significant (Supplementary Fig. 2B). Additionally, the RR of metachronous CRC was 2.66 (95% CI, 0.39–18.13; P=0.317; I2=0%) in the 3 to 4 NAAs group compared to the 1 to 2 NAAs group, and the RR was 1.15 (95% CI, 0.14–9.29; P=0.897; I2=0%) in the ≥5 NAAs group compared to the 3 to 4 NAAs group, which was not statistically significant (Supplementary Table 2).
In summary, there was no statistically significant difference in the RR of metachronous CRC in the ≥3 or ≥5 NAAs group compared to that in the 1 to 2 NAAs group at index colonoscopy; however, the RR of metachronous advanced neoplasia showed a significant increase. Although there was no statistical difference in the RR of metachronous CRC in the ≥5 NAAs group compared to the 3 to 4 NAAs group, the RR of metachronous advanced neoplasia showed an increasing statistical tendency. If studies on high-quality colonoscopy with high-definition endoscopy can be conducted and accumulated over time, meta-analyses should be performed to re-evaluate the risk of metachronous advanced neoplasia and CRC in the 3 to 4 NAAs group.
In patients aged <60 years with ≥10 colorectal adenomas, ≥60 years with ≥20 adenomas, or ≥10 adenomas with a family history of CRC or polyposis, physicians must be careful since these patients have a risk of CRC above the average risk for hereditary CRC syndrome or serrated polyposis syndrome[12]. The USMSTF, despite its weak strength of recommendation and incredibly low evidence, recommends surveillance colonoscopy after 1 year in patients with >10 adenomas removed at high-quality index colonoscopy [10]. In a single-center study conducted in South Korea, which evaluated the metachronous advanced neoplasia in 214 patients with >10 adenomas removed compared to the group with 3 to 10 adenomas removed (n=975), >10 adenomas were an independent risk factor for metachronous advanced neoplasia (OR, 2.25; 95% CI, 1.49–3.38) during a 4.3-year follow-up [39]. Additionally, patients with >10 adenomas have an increased risk of developing familial adenomatous polyposis or MUTYH-associated polyposis [40]. Therefore, genetic testing is recommended considering various factors such as ≥10 adenomas or cumulative lifetime adenomas, age, family history of CRC, and comorbidities (e.g., desmoid tumor, hepatoblastoma, a cribriform-morular variant of papillary thyroid cancer, and congenital hypertrophy of the retinal pigment epithelium) [41,42].
3) Is a Tubulovillous Adenoma or a Villous Adenoma a More Influential Risk Factor That Should Be Considered When Shortening the Colonoscopic Surveillance Interval Compared to a Tubular Adenoma?
Statement 3. Shortening of the colonoscopic surveillance interval should be considered for patients who have tubulovillous or villous adenomas removed at index colonoscopy. (Strength of recommendation: Strong recommendation, Level of evidence: Low)
For histological classification of the adenomas, adenomas with <25% of villous components are classified as tubular adenomas, those with ≥75% of villous components are classified as villous adenomas, and those between the 2 ranges are classified as tubulovillous adenomas [43]. According to the 2020 USMSTF guidelines, similar to the recommendation presented in 2012, removing adenomas with villous histology at index colonoscopy was a risk factor for developing advanced neoplasia in colonoscopic surveillance [10]. Fairley et al. [44] conducted a multicenter cohort study of 3,300 patients with adenomas removed during screening colonoscopy in Pennsylvania, USA. They found that patients with adenomas with villous histology had a higher risk of advanced adenoma and CRC incidence by 3.7-fold and 7.4-fold, respectively, compared to those without villous histology. In a multicenter study conducted in the Netherlands, a cohort of 2,990 patients diagnosed with adenoma during index colonoscopy was followed up for 4 years. As a result, the risk of advanced adenoma in colonoscopic surveillance doubled for patients with villous adenoma compared with those with 1 adenoma on index colonoscopy [45]. In the same study, the risk of advanced adenoma in colonoscopic surveillance was 2.1-fold and 1.7-fold in patients who had 3 adenomas as well as those with adenomas ≥10 mm in size, respectively, suggesting that villous adenoma at index colonoscopy is a risk factor for advanced adenoma in colonoscopic surveillance.
According to a large-scale study in Sweden that evaluated the risk of CRC in patients with colorectal polyps (n=178,377) compared to the general population (n=864,831), the HR of CRC after 6.6 years of follow-up was 1.41 for patients with tubular adenoma, and 2.56 and 3.82 for those with tubulovillous adenoma and villous adenoma, respectively [46]. In a cohort study conducted in the US, 6,161 patients with adenomas were followed for approximately 10 years. Consequently, when patients who had adenomas with villous histology at baseline index colonoscopy were compared to those without polyps, the HR of CRC incidence was 3.17 for tubulovillous adenoma and 8.51 for villous adenoma, showing a remarkably high HR. For those with 1 to 2 adenomas <10 mm in size, the HR of CRC incidence was high 2.91 for adenomas with villous histology; however, the risk of CRC incidence was not significantly high for tubular adenomas [24]. In the same study, the HR of CRC incidence were 3.15, 3.40, and 5.95 for 3 to 10 adenomas, adenomas ≥10 mm in size, and adenomas with high-grade dysplasia, respectively. It can be inferred that adenomas with villous histology at index colonoscopy are a clear risk factor for CRC incidence.
Although the 2013 ESGE guidelines included an adenoma with villous histology at index colonoscopy as a high-risk group for CRC incidence and mortality at long-term follow-up, based on several recent studies reporting that the risk of CRC was not high, an adenoma with villous histology was excluded from the high-risk group in the revised 2020 guidelines [11]. According to a meta-analysis conducted by Saini et al. [47], when tubulovillous adenoma or villous adenoma was removed at baseline index colonoscopy, the RR of advanced adenoma in colonoscopic surveillance after 3 to 4 years of follow-up was not significantly higher compared to those patients who had tubular adenoma removed (RR, 1.26; 95% CI, 0.95–1.66). According to a multicenter, retrospective cohort study conducted in the UK by Atkin et al. [21], the CRC incidence after the removal of villous adenoma at baseline index colonoscopy was not significantly higher than that after the removal of tubular adenoma during the 8-year follow-up period (HR, 1.16; 95% CI, 0.71–1.91). Furthermore, the low level of interobserver consistency among pathologists in diagnosing villous histology in adenomas is also considered an important factor [48].
Similarly, in the UK, due to a low consistency among pathologists in evaluating villous histology, villous histology has not been included in the BSG guidelines from a previous version, and villous histology has been excluded from the definition of advanced adenoma [12,49]. However, the BSG guidelines stated that tubulovillous adenoma or villous adenoma detected at index colonoscopy is a risk factor for advanced adenoma and CRC in the first colonoscopic surveillance and acknowledged consistency in the supporting evidence. It is generally known that the proportions of tubulovillous adenomas and villous adenomas among adenomas are 10% to 15% and 5% to 10%, respectively [50]. A study in the US by He et al. [24] reported the proportion of tubulovillous adenoma and villous adenoma at 19% and 4%, respectively, while a study in the UK conducted by Atkin et al. [21] reported a proportion of 47% and 10%, respectively. The reason for the high proportion of adenomas with villous histology in Atkin et al.’s study is that patients with intermediate-risk adenomas were the participants in their study, and ≥90% of adenomas had a size ≥1 cm in this study. When the ESGE and BSG guidelines excluded adenomas with a villous component from the high-risk group for CRC incidence during surveillance, the study by Atkin et al. [21] was presented as supporting evidence. However, as this study was conducted on patients with intermediate-risk adenomas, and consequently, there was no significant difference in the CRC incidence between the tubular adenoma and villous adenoma groups, careful interpretation of the results is required for these groups. In conclusion, each foreign guideline gives differing perspectives on whether villous tissue is considered a high-risk finding associated with CRC following polypectomy. However, given that the USMSTF still considers villous tissue to be a risk factor and that relatively recent research findings corroborate this assertion, we have opted to include tubulovillous/villous adenoma as risk factors in this guideline.
4) Is a Serrated Polyp a Risk Factor That Should Be Considered When Shortening the Colonoscopic Surveillance Interval?
Statement 4. Colonoscopic surveillance interval should be considered shortening in a patient if a SSL with risk factors was removed during index colonoscopy. (Strength of recommendation: Inconclusive, Level of evidence: Low)
To date, there has been no long-term prospective study on mortality due to CRC and advanced neoplasia in patients with SSLs on index colonoscopy. Macaron et al. [51] reported no evidence supporting an increased risk of advanced neoplasia on surveillance colonoscopy after index colonoscopy with SSLs. However, a case-control study conducted by Erichsen et al. [52] suggested that in patients with an SSL and an SSL with dysplasia on index colonoscopy, the risk of CRC increased by 3-fold and 5-fold, respectively, compared to that in patients with normal index colonoscopy. However, the study findings were limited by the fact that it was unknown whether SSLs were removed on index colonoscopy. In a cohort study by Holme et al. [53], the risk of CRC in patients with serrated polyps >10 mm on index sigmoidoscopy increased by 4.2-fold and 2.5-fold compared to that in patients with no adenoma on index sigmoidoscopy and with no index sigmoidoscopy, respectively. However, this study had limitations because only 81 patients were included. Moreover, it was uncertain whether the results could be extended to SSLs during colonoscopy. Therefore, based on the available results, SSLs on index colonoscopy appear to increase the risk of CRC and advanced neoplasia [24,54-59]. However, the RR and risk factors for CRC and advanced neoplasia compared to tubular adenoma have not been conclusively documented thus far.
TSA [60], SSLs larger than 10 mm [58,61-67], and SSLs with dysplasia have a higher risk for CRC and advanced neoplasia than SSLs without dysplasia and <10 mm [57,68]. Recent guidelines by USMSTF and BSG recommended shortening the colonoscopic surveillance interval if ≥5 SSLs without dysplasia and <10 mm were removed compared to 1 to 4 SSLs without dysplasia and <10 mm [10,12]. Therefore, these factors can be defined as high-risk for CRC and advanced neoplasia if accompanied by a serrated polyp. Each factor is described as follows.
According to previous observational studies, a hyperplastic polyp on index colonoscopy does not increase the risk of CRC or advanced neoplasia. However, these studies are limited because the analyses were not performed according to the size and location of hyperplastic polyps [54,69]. The risk of CRC and advanced neoplasia reportedly did not increase with small hyperplastic polyps in the sigmoid colon and rectum on index colonoscopy compared with normal index colonoscopy [9,70]. However, Schreiner et al. [58] reported that if a hyperplastic polyp or SSL was found in the right colon on index colonoscopy, the risk of synchronous advanced neoplasia increased by 1.9-fold, and the risk of adenoma on surveillance colonoscopy increased by 3.14-fold. Lim et al. [71] reported a 4.8-fold increased risk of advanced neoplasia with a hyperplastic polyp >6 mm in the left colon on index colonoscopy.
5) Is a Traditional Serrated Adenoma a Risk Factor That Should Be Considered When Shortening the Colonoscopic Surveillance Interval?
Statement 5. Shortening of the colonoscopic surveillance interval should be considered for patients who had TSAs removed at index colonoscopy. (Strength of recommendation: Conditional recommenda-tion, Level of evidence: Low)
TSAs are lesions with a risk of developing advanced neoplasia; however, there is a lack of supporting evidence. In a Danish cohort study comparing 2,045 patients with CRC and 8,105 controls, the number of patients with TSAs at baseline index colonoscopy was 14 (0.7%) and 17 (0.2%), respectively, and the adjusted OR was 4.84 (95% CI, 2.36–9.93) [52]. In a prospective cohort study of 12,955 patients aged 50 to 64 years who underwent colonoscopy screening, 81 patients had serrated polyps ≥10 mm in size. The group with large serrated polyps had a higher risk of CRC incidence than the group without polyps (HR, 4.2; 95% CI, 1.3–13.3); thus, large serrated polyps were identified as an independent risk factor for CRC incidence (HR, 3.3; 95% CI, 1.3–8.6) [53]. However, among the 81 patients, only 1 patient was diagnosed with TSA. In a comparative cross-sectional study, the incidence of polyps was compared between 186 patients with TSA and 372 patients with adenoma. The incidence of high-risk adenomas was higher in the TSA group (adjusted OR, 2.37; 95% CI, 1.55–3.63) [60]. To summarize the studies on patients with TSAs discussed above, no previous study has compared only patients with TSAs with the normal group, and even studies that included patients with TSAs had fewer participants. Therefore, the BSG and ESGE guidelines consider TSAs as lesions with a high CRC or advanced neoplasia incidence, as in the case of adenomas, such as serrated polyps ≥10 mm in size and serrated polyps with dysplasia. These guidelines recommend colonoscopic surveillance 3 years after index colonoscopy; however, the risk of TSA is not mentioned separately. In the USMSTF guidelines, colonoscopy surveillance was recommended 3 years after removing TSAs; however, the strength of the recommendation and level of evidence was low. Considering the above recommendations in other guidelines, TSAs are presented as conditional recommendations in this guideline.
6) Is Histology of SSL with Dysplasia a Risk Factor That Should Be Considered When Shortening the Colonoscopic Surveillance Interval?
Statement 6. Shortening the colonoscopic surveillance interval should be considered for patients who had SSLs with dysplasia removed at index colonoscopy. (Strength of recommendation: Conditional recommenda-tion, Level of evidence: Very low)
In a nationwide population-based, nested case-control study in Denmark, the OR for future CRC incidence in SSL with dysplasia was approximately 5-fold compared to the case without polyps (OR, 4.76; 95% CI, 2.59–8.73), the estimated 10-year risk of CRC was 4.43% for patients with SSL with dysplasia, which was higher than the 0.93% in the group without polyps. However, in addition to being a case-control study, another limitation is that since this study was conducted based on pathological findings only, it was not confirmed whether the polyps detected at baseline index colonoscopy were completely removed [52].
In another prospective cohort study, patients with SSLs with dysplasia at baseline index colonoscopy showed a 9-fold higher incidence of metachronous conventional adenomas compared to controls (RR, 9.03; 95% CI, 1.03–16.03); however, there was no significant increase in the development of advanced adenoma (RR, 1.00; 95% CI, 0.15–4.32) [72]. Careful interpretation of the results is required because these are the secondary endpoints of the study, and the number of SSLs with dysplasia was small.
In another retrospective cohort study, high-risk SSLs (SSLs ≥10 mm in size or SSLs with dysplasia) did not correlate with advanced neoplasia at follow-up colonoscopy (HR, 0.57; 95% CI, 0.14–2.30) [55]. However, this study has limitations in that the number of participants with high-risk SSL was only 27, and there was no data on the number of participants with SSLs with dysplasia.
To summarize the evidence discussed above, there is insufficient evidence to determine whether SSLs with dysplasia increase the risk of advanced adenoma and CRC incidence at follow-up colonoscopy. However, SSLs with dysplasia have more histological features consistent with CRC than those without dysplasia. Therefore, we recommend that SSLs with dysplasia be considered high-risk until more evidence is gathered and these patients undergo a repeat colonoscopy within 3 years. Similarly, the USMSTF, ESGE, and BSG guidelines published in 2020 recommended 3-year surveillance colonoscopy for patients with SSLs with dysplasia [10-12].
7) Is the Size of a Serrated Polyp a Risk Factor That Should Be Considered When Shortening the Colonoscopic Surveillance Interval?
Statement 7. Shortening the colonoscopic surveillance in-terval should be considered for patients who were found to have serrated polyps ≥10 mm at index colonoscopy. (Strength of recommendation: Conditional recommenda-tion, Level of evidence: Very low)
According to a population-based RCT (n=100,210) in Norway from 1999 to 2011, when serrated polyps ≥10 mm in size were detected at index colonoscopy, the risk of CRC incidence increased by 4.2-fold compared with those without polyps (HR, 4.2; 95% CI, 1.3–13.3). Moreover, a multivariate logistic regression analysis revealed that a serrated polyp ≥10 mm was an independent risk factor for future CRC incidence (OR, 3.3; 95% CI, 1.3–8.6; P=0.020) [53]. In a cohort study conducted in the US, among 122,899 people who underwent flexible sigmoidoscopy or colonoscopy between 1989 and 2013, when serrated polyps ≥10 mm in size were detected, the risk of CRC incidence increased by 3.35-fold compared to cases without polyps (HR, 3.35; 95% CI, 1.37–8.15; P=0.008) [24]. In another cohort study conducted from 2004 to 2015 in the US among 5,433 patients, when serrated polyps ≥10 mm were detected at index colonoscopy, detection of new serrated polyps ≥10 mm in colonoscopic surveillance showed a significant increase compared to the cases without adenomas or serrated polyps at index colonoscopy (OR, 14.34; 95% CI, 5.03–40.86) [56]. However, in a case-control study conducted from 1998 to 2013 in the US among 2,723 participants, there was no significant difference in future advanced neoplasia incidence between those detected with SSLs ≥10 mm and those with SSLs <10 mm (OR, 1.22; 95% CI, 0.29–5.10) [73]. To summarize the above evidence, shortening of the surveillance period should be considered in patients with serrated polyps ≥10 mm at index colonoscopy because of the increased risk of CRC incidence in colonoscopic surveillance. In the 2020 ESGE and BSG guidelines, serrated polyps ≥10 mm are classified as high-risk, and colonoscopic surveillance is recommended 3 years after polypectomy [11,12]. The USMSTF guidelines classify serrated polyps into different types; for SSLs ≥10 mm, colonoscopic surveillance is recommended after 3 years, whereas for hyperplastic polyps ≥10 mm, colonoscopic surveillance is recommended after 3 to 5 years [10].
8) Is the Number of SSLs a Risk Factor That Should Be Considered When Shortening the Colonoscopic Surveillance Interval?
Statement 8. Shortening the colonoscopic surveillance in-terval may be considered depending on the number of le-sions for a patient detected with a SSL <10 mm in size at in-dex colonoscopy. (Strength of recommendation: Conditional recommenda-tion, Level of evidence: Very low)
To date, there has been insufficient evidence clarifying whether the risk of developing CRC or advanced neoplasia increases when serrated polyps are removed at the index colonoscopy examination. Recent guidelines state that a re-discussion is required on this matter after more research evidence has been gathered [10,12]. According to the USMSTF guidelines published in 2020, for the removal of SSLs <10 mm during index colonoscopy, colonoscopic surveillance should be performed depending on the number of lesions: 5 to 10 years for 1 to 2 lesions, 3 to 5 years for 3 to 4 lesions, and 3 years for 5 to 10 lesions. Additionally, if <20 hyperplastic polyps <10 mm are detected at index colonoscopy, colonoscopic surveillance is recommended after 10 years [10]. Similarly, the BSG guidelines recommend that if there are ≥5 serrated polyps on index colonoscopy, colonoscopic surveillance is recommended after 3 years [12]. To summarize the above evidence, this guideline recommends that colonoscopic surveillance intervals according to the number of SSLs at index colonoscopy be considered based on the strategy for the number of adenomas.
9) Is Piecemeal Resection of Colorectal Polyps ≥20 mm in Size a More Influential Risk Factor, Than En Bloc Resection of the Polyps, That Should Be Considered When Shortening the Colonoscopic Surveillance Interval?
Statement 9. For patients with piecemeal resection of colorectal polyps ≥20 mm in size, shortening the colonoscopic surveillance interval should be considered. (Strength of recommendation: Strong recommendation, Lev-el of evidence: Low)
Piecemeal resection of colorectal polyps has a higher rate of incomplete resection than en bloc resection and is a well-known risk factor for local recurrence after colorectal polyp resection. In a large-scale prospective study of 1,427 patients, the rate of incomplete resection with en bloc resection was reportedly 8.4%, while that with piecemeal resection was considerably higher at 20% [74]. According to a meta-analysis published in 2014, when en bloc resection was performed during endoscopic mucosal resection of non-pedunculated colorectal lesions, the risk of local recurrence was only 3%. However, in cases of piecemeal resection, the risk of recurrence increased to 20% [75]. In particular, the fact that 75% of the local recurrences were detected within 3 months after the procedure indicates the importance of early follow-up in the case of piecemeal resection [75]. According to the results of a large-scale multicenter prospective study by Pellise et al. [76] for serrated lesions ≥20 mm published in 2017, the adjusted HR for recurrence of colorectal lesions increased to 3.4 when piecemeal resection was performed compared to en bloc resection (1.0 vs. 3.4, P=0.002).
In cases of piecemeal resection, the degree of recurrence varies depending on the characteristics of the resected lesions and the procedure. Tate et al. [77] reported that in the case of piecemeal resection for colorectal polyps, the risk of endoscopically determined recurrence after piecemeal resection increased if the polyp size was >40 mm, bleeding occurred during the procedure, or high-grade dysplasia was present. Another study reported that the average period from resection to recurrence decreased as the number of divided lesions increased during piecemeal resection [78]. However, another recent study reported that the risk of recurrence decreased when additional thermal ablation of the post-EMR mucosal defect margin was performed after piecemeal resection of colorectal polyps [79]. Therefore, in the case of piecemeal resection of colorectal polyps, the operator should pay attention to follow-up in the following situations: (1) if the polyp is large, (2) bleeding occurs during the procedure, and (3) in the presence of high-grade dysplasia. Thus, efforts should be made to minimize the number of divided lesions when performing piecemeal resections. Additionally, various approaches should be developed to reduce the risk of recurrence during piecemeal resection.
Based on these studies, most foreign guidelines recommend a short interval of repeat colonoscopy in cases of piecemeal resection of colorectal polyps ≥20 mm in size. The 2020 USMSTF guidelines recommend that in cases of piecemeal resection of adenomas ≥20 mm or SSLs ≥20 mm, the first colonoscopic surveillance should be conducted within 6 months, the second surveillance should be conducted 1 year after the first colonoscopic surveillance, and the third surveillance should be conducted 3 years after the second colonoscopic surveillance [10]. The ESGE guidelines published in 2020 also recommend colonoscopic surveillance within 3 to 6 months after piecemeal resection of colorectal polyps ≥20 mm, similar to the recommendation of the USMSTF guidelines [11]. However, unlike the 2013 ESGE guidelines, where the reference polyp size was set at 10 mm, the reference size of polyps was increased to ≥20 mm according to these guidelines. This is because most of the studies presented as evidence for the recommendation were conducted with polyps ≥20 mm. To summarize, colorectal polyps ≥20 mm in size showed an increasing trend of recurrence rate when piecemeal resection was performed compared to en bloc resection. The extent to which piecemeal resection increases the risk of metachronous advanced neoplasia development remains unclear. However, considering that colorectal polyps ≥20 mm in size increase the risk of CRC in the long-term, the risk of advanced neoplasia development is predicted to increase at recurrence after piecemeal resection. Therefore, in cases of piecemeal resection of colorectal polyps ≥20 mm, colonoscopic surveillance is recommended 6 months after the procedure.
10) Is a Family History of CRC a Risk Factor That Should Be Considered When Shortening the Colonoscopic Surveillance Interval?
Statement 10. Shortening the postpolypectomy colonoscopic surveillance interval is not recommended in patients with a family history of CRC. (Strength of recommendation: Conditional recommenda-tion, Level of evidence: Low)
Several studies have been performed to determine the effect of a family history of CRC on advanced adenoma or CRC incidence. In 2015, Jang et al. [80] conducted a retrospective analysis of the results of colonoscopic surveillance of 434 patients who had advanced adenoma removed; however, the family history of CRC did not increase the risk of developing advanced adenoma during surveillance colonoscopies. In 2016, Park et al. [81] performed a retrospective analysis of the results of colonoscopic surveillance in 1,479 patients with advanced adenoma removed in a multi-institutional study with the participation of 13 Korean hospitals. The results showed that the risk of adenoma or CRC incidence (HR, 0.97; 95% CI, 0.69–1.38; P=0.883) and the risk of developing advanced adenoma (HR, 0.61; 95% CI, 0.23–1.67; P=0.338) showed no statistically significant increase in patients with a family history of CRC than those without. Additionally, in a prospective analysis of 8 studies on the risk of advanced adenoma or CRC incidence by conducting colonoscopic surveillance for 9,167 patients with adenomas removed, no significant correlation was observed between the risk of adenoma or advanced adenoma and family history of CRC [82]. In 2018, Jacobs et al. [83] analyzed the results of colonoscopic surveillance of 7,697 patients with adenomas removed from 8 studies, which included 6 RCTs, and the results did not hint at an increased risk of advanced adenoma or CRC in patients with a family history of CRC (OR, 1.15; 95 % CI, 0.96–1.37).
To summarize the above evidence, most studies that analyzed the relationship between a family history of CRC and the risk of developing advanced adenoma or CRC in colonoscopic surveillance after polyp removal did not present a high level of evidence on the correlation and no statistically significant correlation was observed. Moreover, as most studies either excluded patients with hereditary CRC, such as familial adenomatous polyposis and hereditary nonpolyposis CRC (Lynch syndrome) or were not sufficiently large to include these patients, we hereby specify that this statement is not a recommendation for patients with hereditary CRC. If there is a family history of CRC and related tumors, the possibility of hereditary CRC should be carefully examined, and a colonoscopic surveillance plan should be established accordingly.
2. Timing of Postpolypectomy Surveillance
1) For Patients without CRC-Related High-Risk Findings after Resecting the Polyps, What Is the Appropriate Timing and Interval for Colonoscopic Surveillance?
Statement 11. If there are no high-risk findings related to CRC after complete resection of polyps at high-quality in-dex colonoscopy performed with adequate bowel prepara-tion, colonoscopic surveillance after 5 to 10 years from pol-ypectomy is recommended. However, if the above prereq-uisites are not satisfied or if a high-risk finding of CRC inci-dence was detected after polypectomy before conducting index colonoscopy, the surveillance interval can be short-ened even if no high-risk finding was detected at index colo-noscopy. (Strength of recommendation: Strong recommendation, Level of evidence: Moderate)
In the 2012 Korean guidelines, following a systematic literature search and meta-analysis, an increased risk of developing advanced neoplasia was considered when at least one of the following findings was observed at index colonoscopy after polypectomy, and the cases were defined as having a high-risk finding: ≥3 adenomas, adenomas ≥10 mm in size, tubulovillous or villous adenoma, adenoma with high-grade dysplasia, and serrated polyp ≥10 mm in size [9]. In 2007, Lieberman et al. [84] reported that in the case of <3 tubular adenomas <10 mm at index colonoscopy, the incidence of advanced neoplasia within 5.5 years of the surveillance interval was 4.6%, indicating a higher RR of 1.92 (95% CI, 0.83–4.42) compared to the controls without adenomas; however, the difference was not statistically significant. Compared with controls, however, there was a significant increase in the risk of advanced neoplasia incidence at 11.9% (RR, 5.01; 95% CI, 2.10–11.96) in the case of ≥3 adenomas <10 mm, 15.5% (RR, 6.40; 95% CI, 2.74–14.94) for adenomas ≥10 mm, 16.1% (RR, 6.05; 95% CI, 2.48–14.71) for villous adenoma, and 17.4% (RR, 6.87; 95% CI, 4.61–18.07) for adenomas with high-grade dysplasia [84]. In a single prospective cohort study conducted in South Korea published in 2011 for 3,803 asymptomatic patients aged 50 to 69 years, when <3 adenomas <10 mm were detected at index colonoscopy, the 5-year cumulative incidence of advanced neoplasia showed no significant difference (2.4% vs. 2.0%; HR, 1.14; 95% CI, 0.61–2.17) over controls. However, for adenomas ≥10 mm, ≥3 adenomas, villous adenomas, or adenomas with high-grade dysplasia, the 5-year cumulative incidence of advanced neoplasia was high at 12.2% and the advanced neoplasia developed within 3 to 4 years in most cases [85]. Considered together, the previous guidelines recommended a surveillance interval of 5 years for cases without high-risk findings of advanced neoplasia at index colonoscopy by differentiating from the cases with high-risk findings, but the level of evidence was low.
In many cohort studies conducted since 2012, the risk of CRC incidence and mortality in cases of adenomas without high-risk findings after polypectomy was similar to the normal findings at index colonoscopy [24,28,36,86]. In 2018, Click et al. [28] performed a post-hoc analysis of RCT results on the effect of CRC screening of flexible sigmoidoscopy conducted since 1993. They reported that in the case of adenomas <10 mm without villous adenoma or high-grade dysplasia, there was no significant difference in the risk of developing CRC within 15 years compared to those without adenomas (RR, 1.2; 95% CI, 0.8–1.7; P=0.300). In 2020, He et al. [24] also reported that in the case of adenomas <10 mm in size without villous adenoma or high-grade dysplasia (HR, 1.21; 95% CI, 0.68–2.16; P=0.520) and serrated lesions <10 mm (HR, 1.25; 95% CI, 0.76–2.08; P=0.380), there was no significant difference in the risk of 10-year CRC incidence, respectively, compared to the controls without polyps. In some studies, the risk of CRC incidence and mortality was significantly reduced in the case of adenomas without a high-risk finding after polypectomy at index colonoscopy compared with the general population. This is thought to be because endoscopic resection of adenomas without high-risk findings detected at index colonoscopy contributes to preventing CRC morbidity and mortality [5,22,87]. Additionally, a retrospective cohort study published by He et al. [24] in 2020 reported that adenomas <10 mm in size without villous adenoma or high-grade dysplasia and serrated lesions <10 mm showed no increased risk of 10-year CRC incidence compared with controls without polyps, regardless of the status or number of surveillance colonoscopies. Therefore, there is an increasing body of evidence in recent studies suggesting that surveillance colonoscopy has no additional effect on reducing CRC incidence when there is no CRC-related high-risk finding after polypectomy. Meta-analyses have reported that the incidence of advanced neoplasia in colonoscopic surveillance conducted within 5 years is higher for adenomas without high-risk findings at index colonoscopy than in the normal group; however, the results also include those without statistical significance [88,89].
Individuals without CRC-related high-risk findings after polypectomy are deemed as the group with the same risk level as the normal group in terms of CRC incidence, suggesting that the same colonoscopic surveillance interval could be set for the normal group. Considering that the main purpose of colonoscopic surveillance is to reduce CRC incidence and mortality, this point should be considered as a clear guideline. A colonoscopic surveillance interval of 10 years is generally recommended for the normal group [10]. Therefore, colonoscopic surveillance may be recommended 10 years after polypectomy for individuals with no CRC-related high-risk findings. However, the prerequisite to this recommendation is the complete resection of polyps at high-quality index colonoscopy conducted under adequate bowel preparation. In Korea, considering the relatively low medical costs and high accessibility to healthcare services, the 5-year interval, according to previous guidelines, can serve as an alternative for the transition period to some extent. In the future, if more data on colonoscopic surveillance at 10-year intervals are gathered, the 10-year interval may be established as a recommendation in individuals with no CRC-related high-risk findings following polypectomy. Therefore, colonoscopic surveillance should be conducted 5 to 10 years after polypectomy if there are no high-risk findings related to CRC at index colonoscopy.
2) For Patients with CRC-Related High-Risk Findings after Resection of Polyps, What Is the Appropriate Timing and Interval for Colonoscopic Surveillance?
Statement 12. In the case of CRC-related high-risk findings after polypectomy in high-quality index colonoscopy (ade-noma ≥10 mm; 3 to 5 or more adenomas; tubulovillous ade-noma or villous adenoma; adenoma with high-grade dys-plasia; traditional serrated adenoma; SSL with dysplasia; serrated polyp ≥10 mm; 3 to 5 or more SSLs), shortening of the colonoscopic surveillance interval should be consid-ered by analyzing the specifics of each situation. However, in the case when the above prerequisites have not been sat-isfied or considering the colonoscopy findings prior to inadenodex colonoscopy, the status of adenoma resection, systemic condition of the patient, family history, and medical history of the patient, the surveillance interval can be further adjusted. (Strength of recommendation: Strong recommendation, Level of evidence: Moderate)
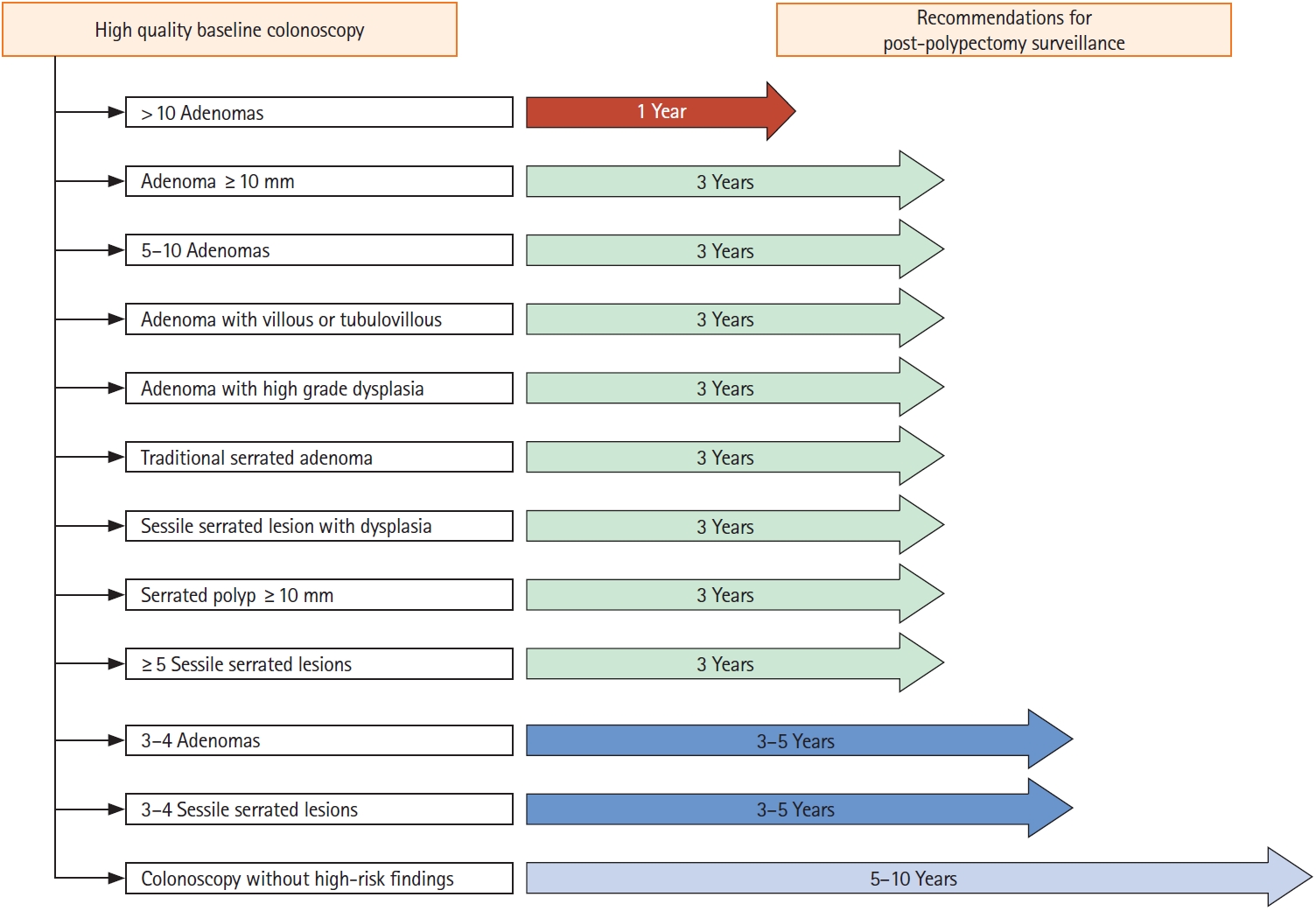
In cases of CRC-related high-risk findings after polypectomy at index colonoscopy, the colonoscopic surveillance interval should be shortened, and such high-risk findings can be determined based on the size, number, and histological findings of resected polyps. In this guideline, the colonoscopic surveillance interval is presented in Table 4 and Fig. 1 according to specific situations.
In a large-scale prospective US cohort study enrolling 15,935 patients detected with polyps at index colonoscopy, the risk of CRC incidence in patients with advanced neoplasia was 13% higher than in those without [28]. In another large cohort study in the US of 3,300 patients, for a high-risk group with adenomas ≥10 mm in size, the risk of advanced neoplasia incidence increased by 3.6-fold (OR, 3.6; 95% CI, 2.8–4.5), and by 5.2-fold for CRC incidence (OR, 5.2; 95% CI, 1.8–15.1) in colonoscopic surveillance [44]. In a large prospective cohort study conducted in Poland that enrolled 236,089 patients with polyps detected at index colonoscopy, the SIR of CRC increased in high-risk groups with the polyps ≥20 mm in size (2.07; 95% CI, 1.40–2.93) or adenomas with high-grade dysplasia (0.79; 95% CI, 0.39–1.41) [22]. According to a multi-institutional prospective study in Korea conducted with 372 patients, the risk of developing advanced neoplasia during colonoscopic surveillance increased by 2.37-fold (OR, 2.37; 95% CI, 1.55–3.63) in the high-risk group with tubulovillous adenoma or villous adenoma at index colonoscopy [60]. According to a cohort study conducted in the US of 5,433 patients, those detected with serrated polyps ≥10 mm at index colonoscopy had a 14.34-fold increase in the risk of serrated polyps ≥10 mm in colonoscopic surveillance (OR, 14.34; 95% CI, 5.03–40.86). However, the risk of developing advanced neoplasia in colonoscopic surveillance did not increase significantly [56]. When the number of adenomas was ≥3 at index colonoscopy, the risk of advanced neoplasia incidence increased by 1.61-fold (OR, 1.61; 95% CI, 1.46–1.78) [12], and the risk of CRC by 4.3-fold (OR, 4.3; 95% CI, 1.40–12.90) [44]. Additionally, when there were 1 to 2 adenomas and 3 to 4 adenomas <10 mm, the incidence of advanced neoplasia in colonoscopic surveillance was similar at 1.4% and 1.8%, respectively, whereas in the case of ≥5 adenomas <10 mm, the advanced neoplasia incidence was 5%. This indicates that with an increase in the number of adenomas, there is a significant increase in the risk of developing advanced neoplasia [29]. Therefore, in cases with high-risk findings of advanced neoplasia at index colonoscopy, colonoscopic surveillance is needed to detect advanced neoplasia and reduce CRC incidence and mortality.
Most high-quality studies related to risk factors for advanced neoplasia incidence are follow-up studies conducted with a cohort of participants in polyp prevention trials. These studies were conducted after the National Polyp Study, and surveillance was performed after the index colonoscopy. In these studies, the risk of advanced adenoma increased in the high-risk group in surveillance conducted 3 years after index colonoscopy, but the actual CRC incidence was rare. Thus, based on the evidence, the adequate timing of surveillance for the high-risk group may be recommended as 3 years. Recent studies compared the colonoscopic surveillance interval of 3 years to the interval within 3 years. In the case of surveillance interval at 3 years, although the incidence of advanced adenoma was high (OR, 2.02; 95% CI, 1.19–3.42), there was no significant difference in terms of CRC incidence and mortality; thus, the appropriate colonoscopic surveillance interval for high-risk groups may be recommended as 3 years [21,90]. However, in many cases, patients who participated in the National Polyp Study and other clinical studies underwent high-quality examination by colonoscopists. Those who had complete resection status of the polyps examined before inclusion in the study underwent a clearing colonoscopy to remove missed polyps. Moreover, considering that many studies excluded patients with a family history of CRC, in determining surveillance timing in clinical practice, it is recommended that in addition to the number or size of adenomas, histological features, and distribution observed at index colonoscopy, detailed information on the quality of colonoscopy examination, family history of CRC, and medical history should also be additionally considered.
Another point to consider is that when the examination of patients shows 2 or more findings among the findings corresponding to the high-risk group that increases the risk of detection of CRC or advanced neoplasia in postpolypectomy colonoscopic surveillance, a few studies have examined whether such cases of multiple findings increase the level of risk. According to a study by Atkin et al. [91] in 1992 using rectosigmoidoscopy, in the high-risk group that had villous adenoma/tubulovillous adenoma or adenomas ≥10 mm in size at the baseline examination, the SIR of CRC for the group with 1 adenoma was 2.9 (95% CI, 1.80–4.50), and for the group with 2 or more adenomas, the CRC SIR increased to 6.6 (95% CI, 3.30–11.80). In 2000, Noshirwani et al. [92] reported that if there were 3 adenomas <10 mm in size at index colonoscopy, the risk of developing advanced adenoma was estimated to be 8.5% in the surveillance conducted after 42 months, and for cases of ≥3 adenomas with the largest of them was ≥10 mm, the risk increased to 21.3%. Additionally, if there were ≥4 adenomas <10 mm in size, the risk of developing advanced adenoma was estimated to be 15.3% in the surveillance, and for cases of ≥4 adenomas, with the largest being ≥10 mm, the risk increased up to 34.5%. According to a study by Anderson et al. [56] in 2018 using colonoscopy, the risk of detecting advanced adenoma in colonoscopic surveillance was 3.86 (95% CI, 2.77–5.39) in the group that had only adenomas with high-grade dysplasia at the baseline examination; however, for the group with both the adenomas with high-grade dysplasia and serrated lesions, the risk increased to 16.04 (95% CI, 6.95–37.00). In a Korean study, the risk of finding advanced adenoma in the surveillance conducted after 3 years was estimated to be 10.7% in the group that was detected to have only adenomas at index colonoscopy, while the risk increased to 17.9% when both adenomas and serrated lesions were detected [93]. These findings indicate that the risk of advanced neoplasia increases if the patient has multiple findings that correspond to the high-risk group. Based on this evidence, if there are 2 or more findings corresponding to the high-risk group, there is a possibility that the surveillance interval should be shortened; however, owing to the lack of research in these areas, it is challenging to recommend a clear guideline for surveillance interval.
DISCUSSION
Patients with colorectal polyps removed have an increased risk of developing colorectal polyps or neoplasia in the future; therefore, management based on appropriate colonoscopic surveillance is required [4,5]. This is a well-established fact over time, and appropriate colonoscopic surveillance is essential for establishing a long-term CRC prevention strategy. To this end, many medical societies in Korea and abroad have developed and distributed guidelines for postpolypectomy colonoscopic surveillance. Since 2020, USMSTF, ESGE, and BSG have completed updating these guidelines. In line with these changes, the Guidelines Development Committee of South Korea presents the interval of postpolypectomy colonoscopic surveillance for Korean patients with colorectal polyps based on the following literature review. At index colonoscopy, if the patient is detected to have at least one of the following, the patient is classified as the high-risk group with an increased risk of future CRC incidence: (1) adenoma ≥10 mm; (2) 3 to 5 or more adenomas; (3) tubulovillous adenoma or villous adenoma; (4) adenoma with high-grade dysplasia; (5) TSA; (6) SSL with dysplasia; (7) serrated polyp ≥10 mm; and (8) 3 to 5 or more SSLs. For the above high-risk groups, we recommend shortening the interval of postpolypectomy colonoscopic sursurveillance; for those without the specified high-risk polypectomy findings, colonoscopic surveillance should be conducted 5 to 10 years after polypectomy.
Adequate bowel preparation and high-quality examination are among the most important factors for establishing an appropriate interval for colonoscopic surveillance. Therefore, in the case of this guideline, unlike previous guidelines, the definitions of adequate bowel preparation and high-quality examination were specified in detail in the process of guideline development. In cases of inadequate examination at index colonoscopy, subsequent colonoscopic surveillance may be needed to complement the initial inadequate examination, which is a significant limitation. According to a large cohort study, an inadequate level of colonoscopy examination at baseline increased the risk of CRC incidence and mortality after polypectomy regardless of colonoscopic surveillance [21]. Inadequate bowel preparation also increases the colonoscopic miss rate of colorectal polyps. Accordingly, most guidelines recommend that the postpolypectomy colonoscopic surveillance interval be applied to patients with adequate bowel preparation [94]. Therefore, adequate bowel preparation and high-quality examinations in clinical practice are crucial, and both must be checked as prerequisites before colonoscopic surveillance.
According to the present guidelines, it was concluded that the risk of advanced neoplasia and CRC incidence increased during colonoscopy surveillance when the number of adenomas at index colonoscopy was 3 to 5 or more. This was confirmed based on the meta-analysis reviewed when preparing the present guidelines as well as the recently revised USMSTF guidelines. As discussed in the previous section, according to the meta-analysis, there was a statistically significant increase in the RR of developing advanced neoplasia in the group that had 3 to 5 or more adenomas removed compared with the group with 1 to 2 adenomas removed. Furthermore, the RR of CRC incidence increased with the number of adenomas, although this increase was not statistically significant. Therefore, in terms of determining the risk of future CRC and advanced neoplasia for evaluating the present guidelines, it was determined that there is a risk of 3 to 5 adenomas or more. The USMSTF guidelines also recommend colonoscopic surveillance 3 to 5 years after developing ≥3 adenomas, although the surveillance interval was slightly longer than in previous guidelines [10]. However, in the BSG and ESGE guidelines, if the number of adenomas is ≥5, the case is considered high-risk [11,12]. This is thought to reflect the results of recently published studies showing that the risk of advanced neoplasia did not significantly increase when the number of adenomas was <5 [21,28]. As more research evidence is gathered in the future, the colonoscopic surveillance interval may change according to the number of adenomas.
A serrated polyp in the colon is an umbrella term used to describe hyperplastic polyps, SSLs, and TSA based on pathological diagnostic criteria [95]. Serrated polyps cause CRC through the serrated pathway rather than carcinogenesis by general adenomas and are currently recognized as important risk factors for CRC and interval CRC [56,96,97]. Therefore, recent international guidelines have added more content to colonoscopic surveillance of these serrated polyps. In the previous ESGE guidelines published in 2013, there was no recommendation regarding serrated polyps. However, in the revised guidelines in 2020, if the size of the serrated polyps is ≥10 mm or if it contains dysplasia, colonoscopic surveillance is recommended after 3 years [11]. The BSG guidelines published in 2020 also specify serrated polyps as premalignant [12]. However, small (1–5 mm) hyperplastic polyps in the rectum were excluded from the classification. In the case of the USMSTF guidelines, categorized repeat colonoscopy intervals are presented for serrated polyps, and the interval is set similar to the surveillance interval for adenomas in general [10]. For SSLs ≥10 mm or those containing dysplasia, the recommended colonoscopic surveillance interval was 3 years. Similarly, when the number of detected SSLs is 5 to 10 or if it contains TSA, it is recommended to conduct colonoscopic surveillance after 3 years. In the case of the present Korean guidelines, adding to the recommendation of previous guidelines in which only serrated polyps ≥10 mm are classified as the high-risk group, SSLs with dysplasia, 3 to 5 or more SSLs, or detection of TSA were also categorized as the high-risk group. For these findings, conducting colonoscopic surveillance after 3 years is recommended.
This revised edition of the Korean guidelines contains new additions compared to previous guidelines, as well as parts that are not specifically stated, as in the case of previous guidelines. The case for piecemeal resection of colorectal polyps is a new addition to this revision. The guidelines published in 2012 had no recommendation on the colonoscopic surveillance interval for piecemeal resection of colorectal polyps [9]. This is because there have been only a few relevant studies in the past, and there was insufficient evidence to recommend a specific colonoscopic surveillance interval. Many studies on the development of metachronous colorectal neoplasia at piecemeal resection were published after 2012 [74-76]. In line with the research evidence, foreign guidelines revised in 2020 all contain recommendations for conducting colonoscopic surveillance 3 to 6 months after piecemeal resection of colorectal polyps ≥20 mm [10-12]. In this revised edition of the guidelines, considering the results of more studies gathered to date and overseas guidelines, although the level of evidence is low, it was judged that the risk of metachronous advanced neoplasia increases in the case of piecemeal resection of colorectal polyps ≥20 mm; therefore, conducting colonoscopic surveillance after 6 months is recommended. Unlike piecemeal resection, the revised guidelines did not present a separate timing for the second colonoscopic surveillance as in the past guidelines. This is because there is a lack of evidence in terms of research on second colonoscopic surveillance, and foreign guidelines also show differences and inconsistencies in their recommendations. Additionally, given the high level of accessibility to colonoscopy in Korea, it is difficult to present specific recommendations for second colonoscopic surveillance at this point.
The age at which postpolypectomy colonoscopic surveillance is stopped differs slightly depending on the guidelines; in most cases, the recommended age is 75 to 80 years [11,12]. However, research on elderly patients regarding the age for stopping postpolypectomy colonoscopic surveillance has provided insufficient evidence. Since this issue is highly dependent on the accessibility of medical resources in each country as well as the health condition of individual patients, there is a limit to applying uniform criteria for all patients. Moreover, since complications from colonoscopy show an increasing trend in the elderly, careful weighing of the possible harm caused by colonoscopy surveillance is important [98,99]. Therefore, the judgment of individual physicians considering the various factors described above is thought to be important in clinical practice, and it is necessary to provide proper explanations regarding the benefits and harms of colonoscopic surveillance for elderly patients.
CONCLUSION
This guideline is a revision of the guidelines published in 2012 and is the second version in Korea. Although some studies on postpolypectomy colonoscopic surveillance have been conducted in Korea since 2012, the available data are still limited in this field. In Western countries, the healthcare environment is fundamentally different from that of Korea, and in particular, there are considerable differences in terms of examination cost and access to colonoscopy. Guidelines greatly influence the clinical practice of physicians, and the scope of the influence is not limited to individual physicians but affects the entire country beyond local communities. Therefore, for Korean guidelines, the utility of medical resources should be considered in the future, and a cost-effectiveness analysis should be performed as a starting point. A cost-effectiveness analysis should be conducted based on research data in Korea in accordance with the domestic healthcare environment, and if necessary, a comparative analysis with other countries should be conducted. The present guidelines are expected to help physicians select more efficient and optimal methods for treating patients in real-world clinical settings. The last point to mention is that since there is a practical limitation to making decisions based only on guidelines considering clinical information of individual patients and various situations, the clinical judgment of individual physicians about the surveillance method and timing derived by synthesizing the guidelines and using various clinical data is thought to be the most important.
Notes
Funding Source
Any costs for literature searches, conferences, and other statistical activities were covered by a research fund provided by the Korean Society of Gastrointestinal Endoscopy (KSGE). The KSGE supported the development of these guidelines. However, this organization did not influence the content of the guidelines.
Conflict of Interest
Park SY is currently serving on the KSGE Publication Committee; however, she was not involved in the peer reviewer selection, evaluation, or decision process of this article. The remaining authors declare no potential conflicts of interest.
Data Availability Statement
Data files are available from Harvard Dataverse: https://doi.org/10.7910/DVN/ZJUSLR
Author Contribution
Conceptualization: Kim SY, Kwak MS. Data curation: Kwak MS. Formal analysis: all authors. Funding acquisition: Byeon JS, Lee OY. Investigation: Kim SY, Kwak MS, Yoon SM, Jung Y, Kim JW, Boo SJ, Oh EH, Jeon SR, Nam SJ, Park SY, Park SK, Chun J, Baek DH, Byeon JS. Methodology: Kwak MS, Choi MY, Park S. Project administration: Byeon JS, Kim HK, Cho JY, Lee MS, Lee OY. Resources: Kim SY, Kwak MS, Yoon SM, Jung Y, Kim JW, Boo SJ, Oh EH, Jeon SR, Nam SJ, Park SY, Park SK, Chun J, Baek DH. Supervision: Byeon JS, Lee OY. Validation: Byeon JS, Lee OY. Visualization: Kim SY, Kwak MS, Yoon SM, Jung Y, Kim JW, Boo SJ, Oh EH, Jeon SR, Nam SJ, Park SY, Park SK, Chun J, Baek DH, Byeon JS, Kim HK, Cho JY, Lee MS, Lee OY. Writing - original draft: Kim SY, Kwak MS, Yoon SM, Jung Y, Kim JW, Boo SJ, Oh EH, Jeon SR, Nam SJ, Park SY, Park SK, Chun J, Baek DH. Writing - review & editing: Byeon JS, Lee OY. Approval of final manuscript: all authors.
Non-Author Contribution
We would like to express our gratitude to Chang Kyun Lee, Dong Il Park, Jae Myung Cha, Young-Eun Joo, Hyun-Soo Kim, Dong Soo Han, Dong-Kyung Chang, and Tae Il Kim, who provided invaluable advice for the development of the Korean Guidelines for Postpolypectomy Colonoscopic Surveillance.
Supplementary Material
Supplementary materials are available at the Intestinal Research website (https://www.irjournal.org).
Supplementary Material 1. Contributors to the guidelines development
ir-2022-00096-Supplementary-1.pdfSupplementary Material 2. Methodology
ir-2022-00096-Supplementary-2.pdfSupplementary Material 3. Search and Selection of Guidelines
ir-2022-00096-Supplementary-3.pdfSupplementary Material 4. Schema illustrating the flow of the search and selection of literature for development of the Guidelines Post-polypectomy Colonoscopic Surveillance
ir-2022-00096-Supplementary-4.pdfSupplementary Material 5. Appraisal
ir-2022-00096-Supplementary-5.pdfSupplementary Material 6. Selected Guidelines
ir-2022-00096-Supplementary-6.pdfSupplementary Material 7. Outline of evidence for recommendations
ir-2022-00096-Supplementary-7.pdfSupplementary Material 8. Evaluation of acceptability and applicability
ir-2022-00096-Supplementary-8.pdfSupplementary Material 9. Flowchart of literature selection process (Ref. PPT)
ir-2022-00096-Supplementary-9.pdfSupplementary Material 10. The outcomes of the external review.
ir-2022-00096-Supplementary-10.pdfSupplementary Fig. 1. The incidence (A) and relative risk (B) of metachronous colorectal advanced neoplasia in the meta-analysis. NAA, non-advanced adenoma; CI, confidence interval; RR, relative risk.
ir-2022-00096-Supplementary-11.pdfSupplementary Fig. 2. The incidence (A) and relative risk (B) of metachronous colorectal cancer in the meta-analysis. NAA, non-advanced adenoma; CI, confidence interval; RR, relative risk.
ir-2022-00096-Supplementary-12.pdfSupplementary Table 1. The Incidence of Metachronous Advanced Neoplasia According to the Number of NAA
ir-2022-00096-Supplementary-13.pdfSupplementary Table 2. The Relative Risk of Metachronous Advanced Neoplasia According to the Number of NAA
ir-2022-00096-Supplementary-14.pdf