 |
 |
- Search
| Intest Res > Volume 20(3); 2022 > Article |
|
Abstract
Early colorectal cancer refers to cancer in the colorectum that is confined to the mucosa or submucosa and does not invade the muscularis propria, irrespective of lymph node or distant metastasis. As the number of persons undergoing screening colonoscopy increases, the proportion of patients diagnosed with precancerous colorectal lesions and early colorectal cancer also increases. In the last decade, innovative optical technologies for endoscopic diagnosis have been introduced and endoscopic treatment techniques such as endoscopic submucosal dissection have provided major breakthroughs in the management of early colorectal cancer. With these remarkable developments, endoscopic treatment has established itself as an alternative to surgical resection in the treatment of early colorectal cancer. This review will discuss the endoscopic diagnosis and treatment of early colorectal cancer. Furthermore, the unmet needs in this field and the latest research addressing those issues will be summarized.
Colonoscopy with polypectomy can reduce the incidence and related mortality of colorectal cancer [1-3]. With the increasing clinical importance of colonoscopy as a screening test, the number of patients diagnosed with early colorectal cancer (ECC) is also increasing. In the last decade, innovative optical technologies for endoscopic diagnosis, such as magnifying endoscopy with narrow-band imaging (NBI), have been introduced, enabling real-time histologic diagnosis and the determination of the depth of invasion of carcinomas [4-8]. Advanced endoscopic treatment techniques, such as endoscopic submucosal dissection (ESD), have provided major breakthroughs in the minimally invasive management of ECC, allowing the successful endoscopic removal of a substantial number of large ECC lesions that cannot be resected en bloc using conventional endoscopic resection techniques [9-11]. This review will address the various endoscopic techniques to determine the indications for endoscopic resection and the different aspects of the application of these techniques in ECC. Furthermore, the unmet needs in the management of ECC and the latest research addressing those issues will be summarized.
ECC refers to cancer in the colorectum that is confined to the mucosa or submucosa and does not invade the muscularis propria, irrespective of lymph node (LN) or distant metastasis. There are differences between East and West in the pathological diagnosis of ECC. In the West, colorectal cancer is defined by invasion through the muscularis mucosa into the submucosa, especially depending on the presence of desmoplasia [12]. On the other hand, in the East, it is defined based on a combination of nuclear and architectural abnormalities, regardless of invasion status. These differences lead to intramucosal carcinoma in the East being diagnosed as high-grade dysplasia in the West, and even intramucosal carcinoma with poorly differentiated is classified as “Tis” in the West. The discrepancies between the West and the East in the diagnosis of ECC can be addressed by active East-West exchanges. Ultimately, a pathologist should make a logical and consistent histologic diagnosis, evaluate various risk factors for metastasis, and convey it to the clinicians. Similar to the treatment principle for other cancers, the goal of endoscopic treatment for ECC is the complete removal of cancer cells, which is necessary for cure.
Intramucosal cancer is almost never associated with LN metastasis, and complete removal of cancer can be achieved if the primary tumor is completely removed with endoscopic resection. However, when the cancer has invaded the submucosal layer, LN metastasis is observed in approximately 10% of the cases [13]. The current guidelines state that a depth of submucosal invasion of > 1 mm, lymphovascular invasion, intermediate- to high-grade tumor budding, and poor differentiation are unfavorable histologic features suggestive of LN metastasis in ECC [14-16].
The classification of the depth of submucosal invasion differs according to the morphology of ECC: pedunculated ECC has been classified using the Haggitt classification [17], whereas non-pedunculated ECC has been classified using the Kudo or Kikuchi classification [18,19]. In a retrospective study in patients with colorectal cancer who underwent intestinal resection, the proportion of patients with LN metastasis was 3% for Kudo sm1 cases, 8% for Kudo sm2 cases, and 23% for Kudo sm3 cases [20]. Another study reported that when the Haggitt system was used in categorizing the depth of submucosal invasion in ECC, the proportion of patients with LN metastasis was 2.4% for Haggitt 1/2 cases and 13.0% for Haggitt 3/4 cases [21]. In these classifications, the deeper invasion, the higher the risk of LN metastasis [16-18]. However, these classification systems require measuring the relative depth of invasion after resecting the entire submucosal layer. Therefore, for endoscopic resection specimens, a method of measuring the absolute depth of invasion is widely applied. A multicenter study conducted in Japan analyzed the rate of LN metastasis according to the absolute depth of invasion in patients with submucosal invasive colorectal cancer. The findings indicated that both patients with pedunculated ECC with an invasion depth of < 3 mm, in the absence of lymphatic invasion, and patients with non-pedunculated ECC with an invasion depth of < 1 mm had an LN metastasis rate of 0% [22]. In a retrospective study analyzing the pathologic findings of patients with ECC who underwent endoscopic resection or surgery, no LN metastasis was observed when the depth of submucosal invasion was limited to 1 mm. However, when the depth of invasion reached up to 1,500 and 2,000 µm, LN metastasis was observed in 0.5% and 1.5% of patients, respectively. A meta-analysis of 17 studies revealed that a submucosal invasion depth of ≥ 1 mm is a strong predictor of LN metastasis in ECC (relative risk, 5.2; 95% confidence interval, 1.8-15.4) [23]. Therefore, for non-pedunculated ECC, a depth of submucosal invasion of ≥ 1 mm from the muscularis mucosae is associated with the risk of LN metastasis and is the widely accepted cutoff value for deep submucosal invasion [24-26]. However, some studies have reported that assessing the risk of metastasis based on the absolute depth of invasion has a low predictive power [23,24]; thus, additional methods are needed.
Many studies have demonstrated that lymphovascular invasion is an independent risk factor for LN metastasis [6,27,28]. To date, several meta-analysis studies have consistently reported that the presence of lymphatic and vascular invasion was significantly associated with an increased risk of LN metastasis in patients with ECC [23,29]. Tumor budding is defined as the presence of a single tumor cell or a cluster of fewer than 5 tumor cells at the tumor-invasive front of the resected specimen, which has been recently accepted as an international consensus system for the reporting, scoring, and assessment of tumor budding in colorectal cancer [30,31]. The scoring system for tumor budding differs across studies; however, in general, the presence of 5 or more tumor buds is classified as intermediate to high risk and has been revealed in several studies to be an independent risk factor for LN metastasis in ECC [24,32-34]. Poor histologic types, including poorly differentiated adenocarcinoma, signet ring cell carcinoma, and mucinous carcinoma, are traditional predictors of LN metastasis in ECC [24,33]. Previous studies have shown that poor histologic types have a considerably higher risk of LN metastasis than well-differentiated types [14,27,35].
For submucosal colorectal cancer with a high risk of LN metastasis, endoscopic resection has a higher recurrence rate than surgical resection. Therefore, the current guidelines recommend an additional surgery when the abovementioned unfavorable histologic features are observed after endoscopic resection. A Japanese collaborative study in 2004 investigated the relationship between LN metastasis and several histopathologic features according to the morphology of lesions and the absolute depth of invasion in submucosal invasive colorectal cancer [22]. The findings showed that in the absence of lymphatic invasion, no LN metastasis was observed in pedunculated ECC with head invasion and stalk invasion < 3 mm. However, even in the presence of histologic features such as lymphatic invasion, venous invasion, and spurting, none of the cases with a submucosal invasion depth of < 1 mm showed LN metastasis [22]. These results suggest that assessing the risk of LN metastasis in ECC based on the presence or absence of a single unfavorable histologic feature has limitations. Hence, a comprehensive risk evaluation system for LN metastasis in ECC is required. Follow-up studies and complementary explanations on this topic are covered later in this review.
For endoscopic resection of ECC with curative intent, it is crucial to determine the possibility of LN metastasis. Radiologic examinations such as computed tomography may be performed to determine whether LN metastasis is present. However, as this method simply evaluates the presence of metastasis based on the increase in LN size, it has a low sensitivity [36]. Therefore, radiologic examinations have limited value in determining the indications for endoscopic resection of ECC.
As mentioned above, the risk of LN metastasis in ECC is positively related to the depth of submucosal invasion. Therefore, it is essential to estimate the depth of invasion by performing a colonoscopy before deciding the treatment (endoscopic or surgical resection) of ECC. A variety of endoscopic evaluation methods for estimating the invasion depth and therefore for predicting LN metastasis have been developed, including magnifying chromoendoscopy and NBI.
The Kudo pit pattern classification system is an effective tool for estimating the depth of submucosal invasion in ECC (Table 1) [37,38]. The type V, particularly VN, pit pattern on chromoendoscopy suggests a high probability of deep submucosal invasion [39]. According to a meta-analysis of 17 studies that investigated the diagnostic performance of magnifying chromoendoscopy, this method had a sensitivity of 81% and a specificity of 95% in distinguishing deep submucosal invasion [40].
NBI is used to assess the vascular pattern and surface structure of the colonic mucosa. In particular, the following NBI findings indicate deep submucosal invasion: completely unclear or amorphous surface pattern, severely irregular thickness and arrangement of capillary vessels, and avascular or loose microvessel areas [41-44]. Several NBI classification systems have been proposed to aid in the diagnosis of colorectal tumors and the assessment of submucosal invasion. These include Sano’s classification, Hiroshima’s classification, the more recent NBI International Colorectal Endoscopic classification, and the Japan NBI Expert Team classification (Table 2) [45,46]. In a meta-analysis of 13 studies evaluating the accuracy of NBI in diagnosing deep submucosal invasion, the method showed a sensitivity of 77% and a specificity of 98% [40].
In addition to pit pattern analysis and NBI, white-light endoscopy provides macroscopic findings that suggest deep submucosal invasion, including hardness of a lesion, presence of deep depressions or ulcers, fold convergence, and non-lifting sign [47].
Therefore, it is essential to assess the macroscopic findings, pit pattern, and vascular/surface pattern using colonoscopy to estimate the depth of submucosal invasion before performing endoscopic resection of ECC. If deep submucosal colorectal cancer is strongly suspected based on the endoscopic findings, primary surgery should be performed. However, as these evaluation tools cannot completely replace histopathologic assessment, additional surgery should be considered when histopathologic findings highly suggestive of LN metastasis are confirmed even after complete endoscopic resection of ECC.
To achieve curative endoscopic resection of ECC, en bloc resection is essential. A meta-analysis of 33 studies evaluating local recurrence after the endoscopic resection of non-pedunculated polyps showed that the local recurrence rate was 3% when en bloc resection was performed but reached up to 20% in cases of piecemeal resection [48]. In addition, piecemeal resection makes tissue reconstruction difficult, which limits the pathologic evaluation of unfavorable histologic features of ECC.
Snare polypectomy is divided into hot snare polypectomy (HSP) and cold snare polypectomy (CSP) depending on whether a high-frequency generator is used. As HSP is associated with thermal injury and can cause intestinal perforation, CSP has recently been preferred [49]. A recent study compared the resection rates and safety profile of CSP and HSP in polyps measuring 5-10 and 11-20 mm [50]. The results provided more evidence that CSP is not inferior to HSP for adenomas with a diameter of 5-10 mm and that CSP should be favored considering its safety and cost-effectiveness [50]. However, other recent studies suggested that CSP results in a more superficial resection than HSP or endoscopic mucosal resection (EMR) [51,52] and can result in positive vertical margins and recurrence in small early invasive tumors. Although invasive colorectal lesions < 10 mm are uncommon, it is important to note that CSP is not appropriate for suspected malignancy because of the possibility of vertical margin involvement. In summary, CSP is becoming the standard treatment for benign-looking diminutive and small sessile polyps; however, it is not suitable for malignant lesions.
EMR, one of the most commonly used endoscopic resection methods, combines the classic principles of conventional snare polypectomy with submucosal fluid injection. To minimize bleeding during the procedure, diluted epinephrine-saline solution (1:100,000) can be injected into the submucosa via a needle. A diluted dye, such as inert indigo carmine or methylene blue, can be used to outline the extent of the submucosal cushion and to confirm that the resection is on the proper plane.
EMR can remove colorectal tumors with sufficient lateral and vertical margins, with a relatively low risk of perforation after the procedure. Despite these advantages, the main limitation of EMR is the high probability of piecemeal resection when removing a large polyp ( ≥ 20 mm), which is associated with a risk of recurrence of approximately 12%-20% [48,53]. In particular, for large non-pedunculated polyps, piecemeal resection makes it difficult for the pathologist to comment on the completeness of the resection; thus, EMR is not appropriate as an endoscopic resection technique for ECC. If ECC is suspected, it should be determined whether EMR is the optimal technique for curative resection considering the morphology and size of the lesion. In general, when the size of the lesion is < 2 cm, en bloc resection can be achieved with EMR; however, caution should be taken when this method is attempted for ECC. In contrast, when the size of the lesion is > 2 cm or when it is difficult to achieve en bloc resection with EMR based on the morphology and location of the lesion, ESD should be considered.
ESD, which is the newest endoscopic resection technique, can be used for curative resection of large superficial neoplasms in the gastrointestinal tract. It has an advantage over typical EMR in that ESD enables the en bloc removal of lesions > 20 mm in size, thus avoiding piecemeal resection, which is linked to local recurrence [54]. ESD is a challenging procedure from a technical standpoint because of the narrow space in the colon, difficult positioning of the scope, thin bowel walls, and presence of colonic folds. ESD is mainly indicated for early invasive lesions localized in the mucosa or superficial submucosal layer with a diameter of > 20 mm that cannot be resected en bloc with EMR. ESD may also be considered for superficial submucosal invasive cancer < 20 mm in size with substantial submucosal fibrosis [55]. The current guidelines recommend ESD for the removal of colonic neoplasms highly suspected of superficial submucosal invasion, particularly if the lesion is > 20 mm, or considering ESD for colorectal lesions that otherwise cannot be optimally and radically removed using snare-based techniques [55]. However, procedure-related complications more frequently occur in ESD than in EMR [56]. The major complications of ESD include bleeding and perforation. Most cases of perforation during the procedure are minor and can be closed using an endoscopic clip, and the number of cases requiring emergency surgery is relatively low (approximately 0.5%) [57,58]. Delayed bleeding is observed in approximately 2.0% of cases [54,58,59].
In summary, ESD has a higher en bloc resection rate than conventional EMR; however, it has a high complication rate and long procedure time and requires a skilled endoscopist. Therefore, it is important to understand the appropriate indications for ESD based on the guidelines and to apply them to clinical practice. Meanwhile, endoscopic resection is a good treatment option for pedunculated polyps with features of submucosal invasion, as the endoscopic procedure is relatively simple and safe and the overall histological features may still be favorable [16,60]. En bloc resection through the stalk resection can be achieved in all pedunculated colorectal polyps. We presented an algorithm for the approach to malignant polyp by the morphology of the lesion in Fig. 1 [16].
Although some ECC cases can be successfully treated using endoscopic resection techniques, approximately 70%-80% of patients require radical surgery to achieve a complete cure, owing to the possibility of LN metastasis on pathologic analysis. However, the rate of pathologically confirmed LN metastasis after surgical resection was estimated to be approximately 10% in patients whose endoscopic resection specimens showed unfavorable histologic features [20,61]. In other words, a substantial number of patients undergo unnecessary surgery [23,57,59,62]. Various attempts are being made to overcome the shortcomings of the current guidelines and to increase the accuracy of the prediction of LN metastasis in ECC.
As mentioned above, it is challenging to predict the exact risk of LN metastasis after the endoscopic resection of ECC. To overcome this difficulty, several attempts have been made to comprehensively estimate the actual incidence of LN metastasis based on the combination of unfavorable histologic features relevant to LN metastasis [63-65]. The findings indicated that when 4 histologic features (e.g., depth of invasion > 1,000 µm, lymphovascular invasion, tumor budding grade 2/3, and poor histologic differentiation) were present, the risk of LN metastasis reached 34.1%; however, in cases with a depth of invasion of > 1,000 µm but without any other histologic factors, the risk of LN metastasis was only 1.6%.64 In another study, the combined presence of deep submucosal invasion and lymphovascular invasion resulted in a risk of LN metastasis of 22%, and adding poor histologic differentiation increased the risk to 71.4%. Meanwhile, when only deep submucosal invasion was present without other histologic factors, the risk of LN metastasis was only 1.7% [63]. We summarized the results of these studies and presented them in Table 3. Although the detailed results of the 2 studies were different, they consistently showed that when there was only deep submucosal invasion without other histologic factors in ECC, the risk of LN metastasis was relatively low. Also, in both studies, the presence of 2 or more adverse histological factors appeared to increase the risk of LN metastasis further than the presence of only 1 factor. More follow-up studies are needed to enable the evaluation of the risk of LN metastasis in ECC through a comprehensive risk stratification rather than by depending on a single histologic factor.
An artificial intelligence (AI) model using various parameters, including high-risk histopathologic findings, was developed to predict LN metastasis in ECC [66,67]. In a recent study performed in Japan, an AI model using 8 clinical and histologic variables showed superior results in predicting LN metastasis in ECC compared with the U.S. and Japanese guidelines [67]. To keep up with this trend, our institution developed an AI model for predicting LN metastasis in ECC that integrates endoscopic findings suggestive of deep submucosal invasion and unfavorable histologic findings. Our AI-based model showed superior ability in predicting LN metastasis in ECC compared with the current Japanese guideline (area under the curve, 0.764 vs. 0.606; unpublished data).
Meanwhile, estimating the depth of submucosal invasion in ECC is a key to determining the indications for endoscopic resection. Recently, an AI-enhanced attention-guided whitelight colonoscopy system was developed to differentiate noninvasive or superficially invasive neoplasms from deeply invasive colorectal cancer. It showed an overall accuracy of 91.1% with a sensitivity of 91.2% and a specificity of 91.0% [68]. Although technical limitations remain with respect to clinical application and such AI models cannot outperform experienced endoscopists, these tools are expected to aid the clinical decision-making process in the treatment of ECC in the future.
With accumulating evidence indicating that the expression patterns of microRNAs (miRNAs) derived from tissues reflect the pathologic status of cancer, attempts have been made to predict LN metastasis using biomarkers in ECC. By analyzing miRNA sequencing data in tissues derived from patients with ECC with or without LN metastasis, recent studies discovered a novel genetic biomarker that shows a different expression pattern according to the presence or absence of LN metastasis [69,70]. Furthermore, a preoperative risk stratification model for LN metastasis was developed by combining key clinical features and a novel transcriptomic biomarker panel (5 messenger RNAs, AMT, FOXA1, PIGR, MMP1, and MMP9; 4 miRNAs, miR-181b, miR-193b, miR-195, and miR-411) assessed using liquid biopsy of blood [71]. This blood-based, non-invasive model demonstrates high accuracy in predicting LN metastasis in ECC and is expected to contribute to reducing unnecessary surgery in patients with this malignancy. Further studies investigating useful molecular biomarkers are warranted.
In recent years, the diagnosis rate of ECC has increased owing to the implementation of mass screening for colorectal cancer and frequent health examinations. In addition, innovative optical technologies for endoscopic diagnosis (e.g., NBI) have been introduced, offering the possibility of real-time histologic diagnosis and facilitating the determination of the depth of cancer invasion. In particular, predicting the possibility of deep submucosal invasion of ECC using these endoscopic technologies is an essential competency of a skilled endoscopist. Meanwhile, when considering endoscopic resection for ECC, it is crucial to ensure sufficient tumor-free resection margins along with en bloc resection. Thus, endoscopists should determine the appropriate endoscopic resection method according to the location, size, and morphology of the colorectal lesion. In addition, endoscopists should be aware of unfavorable histologic findings suggestive of LN metastasis, which require additional surgery after endoscopic resection. Currently, various attempts are being made to enable more precise clinical decision-making with respect to the diagnosis and treatment of ECC, and it is expected that more accurate guidelines will be developed in the future.
ADDITIONAL INFORMATION
Fig. 1.
Management algorithm of colorectal polyp according to morphology and estimated depth of invasion. JNET, Japan Narrow-Band Imaging (NBI) Expert Team.
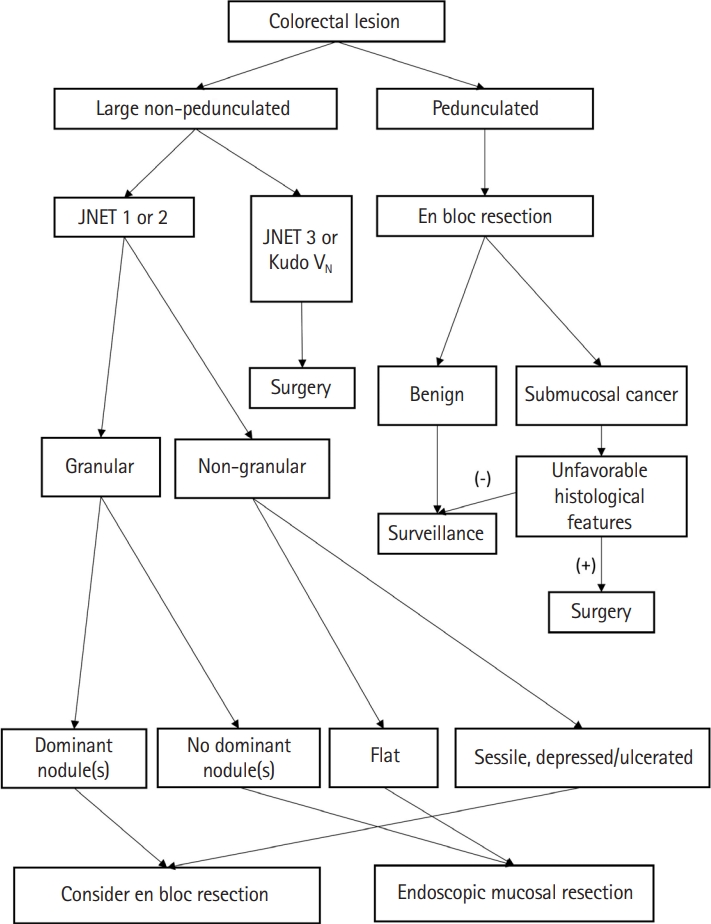
Table 1.
Kudo Classification of Pit Pattern[38]
Table 2.
Japanese NBI Expert Team Classification[45]
Table 3.
Correlation between Single or Multiple Unfavorable Histologic Features and Risk of LN Metastasis in Early Colorectal Cancer
| Histologic features | Suh et al. (2012) [63] | Yasue et al. (2019) [64] | ||
|---|---|---|---|---|
| No. | LN metastasis, No. (%) | No. | LN metastasis, No. (%) | |
| [1] Depth of invasion > 1 mm or sm2/sm3 | 118 | 2 (1.7) | 258 | 4 (1.6) |
| [2] Lymphovascular invasion | 43 | 9(20.9) | 20 | 3 (15.0) |
| [3] Poor differentiation | 1 | 0 | 3 | 0 |
| [4] Tumor budding grade 2/3 | NA | NA | 1 | 0 |
| [1] + [2] | 100 | 22(22.0) | 189 | 24 (12.7) |
| [2] + [3] | 1 | 1 (100) | 1 | 0 |
| [1] + [3] | 1 | 1 (100) | 15 | 1 (6.3) |
| [1] + [4] | NA | NA | 40 | 3 (7.5) |
| [2] + [4] | NA | NA | 1 | 0 |
| [3] + [4] | NA | NA | 1 | 0 |
| [1] + [2] + [3] | 7 | 5(71.4) | 17 | 5 (29.4) |
| [1] + [2] + [3] + [4] | NA | NA | 44 | 15 (34.1) |
REFERENCES
1. Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012;366:687-696.



2. Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med 2013;369:1106-1114.


3. Pilonis ND, Bugajski M, Wieszczy P, et al. Long-term colorectal cancer incidence and mortality after a single negative screening colonoscopy. Ann Intern Med 2020;173:81-91.


4. Tung SY, Wu CS, Su MY. Magnifying colonoscopy in differentiating neoplastic from nonneoplastic colorectal lesions. Am J Gastroenterol 2001;96:2628-2632.


5. Tamura S, Ookawauchi K, Onishi S, et al. The usefulness of magnifying chromoendoscopy: pit pattern diagnosis can predict histopathological diagnosis precisely. Am J Gastroenterol 2002;97:2934-2935.


6. Tischendorf JJ, Wasmuth HE, Koch A, Hecker H, Trautwein C, Winograd R. Value of magnifying chromoendoscopy and narrow band imaging (NBI) in classifying colorectal polyps: a prospective controlled study. Endoscopy 2007;39:1092-1096.


7. Adler A, Pohl H, Papanikolaou IS, et al. A prospective randomised study on narrow-band imaging versus conventional colonoscopy for adenoma detection: does narrow-band imaging induce a learning effect? Gut 2008;57:59-64.


8. Rastogi A, Bansal A, Wani S, et al. Narrow-band imaging colonoscopy: a pilot feasibility study for the detection of polyps and correlation of surface patterns with polyp histologic diagnosis. Gastrointest Endosc 2008;67:280-286.


9. Yamamoto H. Endoscopic submucosal dissection of early cancers and large flat adenomas. Clin Gastroenterol Hepatol 2005;3(7 Suppl 1):S74-S76.


10. Deprez PH, Bergman JJ, Meisner S, et al. Current practice with endoscopic submucosal dissection in Europe: position statement from a panel of experts. Endoscopy 2010;42:853-858.


11. Draganov PV, Wang AY, Othman MO, Fukami N. AGA Institute Clinical Practice Update: endoscopic submucosal dissection in the United States. Clin Gastroenterol Hepatol 2019;17:16-25.


12. Yao T, Shiono S. Differences in the pathological diagnosis of colorectal neoplasia between the East and the West: present status and future perspectives from Japan. Dig Endosc 2016;28:306-311.


13. Park YJ, Kim WH, Paeng SS, Park JG. Histoclinical analysis of early colorectal cancer. World J Surg 2000;24:1029-1035.



14. Beaton C, Twine CP, Williams GL, Radcliffe AG. Systematic review and meta-analysis of histopathological factors influencing the risk of lymph node metastasis in early colorectal cancer. Colorectal Dis 2013;15:788-797.


15. Hashiguchi Y, Muro K, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 2020;25:1-42.




16. Shaukat A, Kaltenbach T, Dominitz JA, et al. Endoscopic recognition and management strategies for malignant colorectal polyps: recommendations of the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2020;159:1916-1934.


17. Haggitt RC, Glotzbach RE, Soffer EE, Wruble LD. Prognostic factors in colorectal carcinomas arising in adenomas: implications for lesions removed by endoscopic polypectomy. Gastroenterology 1985;89:328-336.


18. Kikuchi R, Takano M, Takagi K, et al. Management of early invasive colorectal cancer: risk of recurrence and clinical guidelines. Dis Colon Rectum 1995;38:1286-1295.

19. Moss A, Bourke MJ, Williams SJ, et al. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology 2011;140:1909-1918.


20. Nascimbeni R, Burgart LJ, Nivatvongs S, Larson DR. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum 2002;45:200-206.


21. Barel F, Cariou M, Saliou P, et al. Histopathological factors help to predict lymph node metastases more efficiently than extranodal recurrences in submucosa invading pT1 colorectal cancer. Sci Rep 2019;9:8342.




22. Kitajima K, Fujimori T, Fujii S, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol 2004;39:534-543.



23. Bosch SL, Teerenstra S, de Wilt JH, Cunningham C, Nagtegaal ID. Predicting lymph node metastasis in pT1 colorectal cancer: a systematic review of risk factors providing rationale for therapy decisions. Endoscopy 2013;45:827-834.


24. Ueno H, Hase K, Hashiguchi Y, et al. Novel risk factors for lymph node metastasis in early invasive colorectal cancer: a multi-institution pathology review. J Gastroenterol 2014;49:1314-1323.



25. Choi JY, Jung SA, Shim KN, et al. Meta-analysis of predictive clinicopathologic factors for lymph node metastasis in patients with early colorectal carcinoma. J Korean Med Sci 2015;30:398-406.




26. Kim B, Kim EH, Park SJ, et al. The risk of lymph node metastasis makes it unsafe to expand the conventional indications for endoscopic treatment of T1 colorectal cancer: a retrospective study of 428 patients. Medicine (Baltimore) 2016;95:e4373.


27. Al-Sukhni E, Attwood K, Gabriel EM, LeVea CM, Kanehira K, Nurkin SJ. Lymphovascular and perineural invasion are associated with poor prognostic features and outcomes in colorectal cancer: a retrospective cohort study. Int J Surg 2017;37:42-49.


28. Han J, Hur H, Min BS, Lee KY, Kim NK. Predictive factors for lymph node metastasis in submucosal invasive colorectal carcinoma: a new proposal of depth of invasion for radical surgery. World J Surg 2018;42:2635-2641.



29. Wada H, Shiozawa M, Katayama K, et al. Systematic review and meta-analysis of histopathological predictive factors for lymph node metastasis in T1 colorectal cancer. J Gastroenterol 2015;50:727-734.



30. Ueno H, Murphy J, Jass JR, Mochizuki H, Talbot IC. Tumour ‘budding’ as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology 2002;40:127-132.



31. Cappellesso R, Luchini C, Veronese N, et al. Tumor budding as a risk factor for nodal metastasis in pT1 colorectal cancers: a meta-analysis. Hum Pathol 2017;65:62-70.


32. Masaki T, Matsuoka H, Sugiyama M, Abe N, Sakamoto A, Atomi Y. Actual number of tumor budding as a new tool for the individualization of treatment of T1 colorectal carcinomas. J Gastroenterol Hepatol 2006;21:1115-1121.


33. Choi PW, Yu CS, Jang SJ, Jung SH, Kim HC, Kim JC. Risk factors for lymph node metastasis in submucosal invasive colorectal cancer. World J Surg 2008;32:2089-2094.



34. Yim K, Won DD, Lee IK, Oh ST, Jung ES, Lee SH. Novel predictors for lymph node metastasis in submucosal invasive colorectal carcinoma. World J Gastroenterol 2017;23:5936-5944.



35. Mou S, Soetikno R, Shimoda T, Rouse R, Kaltenbach T. Pathologic predictive factors for lymph node metastasis in submucosal invasive (T1) colorectal cancer: a systematic review and meta-analysis. Surg Endosc 2013;27:2692-2703.



36. Zerhouni EA, Rutter C, Hamilton SR, et al. CT and MR imaging in the staging of colorectal carcinoma: report of the Radiology Diagnostic Oncology Group II. Radiology 1996;200:443-451.


37. Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc 1996;44:8-14.


38. Tanaka S, Kaltenbach T, Chayama K, Soetikno R. High-magnification colonoscopy (with videos). Gastrointest Endosc 2006;64:604-613.


39. Hurlstone DP, Cross SS, Adam I, et al. Endoscopic morphological anticipation of submucosal invasion in flat and depressed colorectal lesions: clinical implications and subtype analysis of the kudo type V pit pattern using high-magnification-chromoscopic colonoscopy. Colorectal Dis 2004;6:369-375.


40. Backes Y, Moss A, Reitsma JB, Siersema PD, Moons LM. Narrow band imaging, magnifying chromoendoscopy, and gross morphological features for the optical diagnosis of T1 colorectal cancer and deep submucosal invasion: a systematic review and meta-analysis. Am J Gastroenterol 2017;112:54-64.



41. Hirata M, Tanaka S, Oka S, et al. Evaluation of microvessels in colorectal tumors by narrow band imaging magnification. Gastrointest Endosc 2007;66:945-952.


42. Wada Y, Kudo SE, Kashida H, et al. Diagnosis of colorectal lesions with the magnifying narrow-band imaging system. Gastrointest Endosc 2009;70:522-531.


43. Hewett DG, Kaltenbach T, Sano Y, et al. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology 2012;143:599-607.


44. Gonai T, Kawasaki K, Nakamura S, et al. Microvascular density under magnifying narrow-band imaging endoscopy in colorectal epithelial neoplasms. Intest Res 2020;18:107-114.




45. Sumimoto K, Tanaka S, Shigita K, et al. Clinical impact and characteristics of the narrow-band imaging magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Gastrointest Endosc 2017;85:816-821.


46. Sano Y, Hirata D, Saito Y. Japan NBI Expert Team classification: narrow-band imaging magnifying endoscopic classification of colorectal tumors. Dig Endosc 2018;30:543-545.



47. Ishiguro A, Uno Y, Ishiguro Y, Munakata A, Morita T. Correlation of lifting versus non-lifting and microscopic depth of invasion in early colorectal cancer. Gastrointest Endosc 1999;50:329-333.


48. Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014;46:388-402.


49. Piraka C, Saeed A, Waljee AK, Pillai A, Stidham R, Elmunzer BJ. Cold snare polypectomy for non-pedunculated colon polyps greater than 1cm. Endosc Int Open 2017;5-E184-E189.

50. Gessl I, Waldmann E, Penz D, et al. Resection rates and safety profile of cold vs. hot snare polypectomy in polyps sized 5-10 mm and 11-20 mm. Dig Liver Dis 2019;51:536-541.


51. Ito A, Suga T, Ota H, Tateiwa N, Matsumoto A, Tanaka E. Resection depth and layer of cold snare polypectomy versus endoscopic mucosal resection. J Gastroenterol 2018;53:1171-1178.



52. Suzuki S, Gotoda T, Kusano C, et al. Width and depth of resection for small colorectal polyps: hot versus cold snare polypectomy. Gastrointest Endosc 2018;87:1095-1103.


53. De Ceglie A, Hassan C, Mangiavillano B, et al. Endoscopic mucosal resection and endoscopic submucosal dissection for colorectal lesions: a systematic review. Crit Rev Oncol Hematol 2016;104:138-155.


54. Fuccio L, Hassan C, Ponchon T, et al. Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: a systematic review and meta-analysis. Gastrointest Endosc 2017;86:74-86.


55. Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015;47:829-854.


56. Odagiri H, Yasunaga H. Complications following endoscopic submucosal dissection for gastric, esophageal, and colorectal cancer: a review of studies based on nationwide large-scale databases. Ann Transl Med 2017;5:189.



57. Messmann H, Probst A. Management of endoscopic submucosal dissection complications. Endoscopy 2009;41:712-714.


58. Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010;72:1217-1225.


59. Akintoye E, Kumar N, Aihara H, Nas H, Thompson CC. Colorectal endoscopic submucosal dissection: a systematic review and meta-analysis. Endosc Int Open 2016;4:E1030-E1044.

60. Puig I, López-Cerón M, Arnau A, et al. Accuracy of the Narrow-Band Imaging International Colorectal Endoscopic classification system in identification of deep invasion in colorectal polyps. Gastroenterology 2019;156:75-87.

61. Tanaka S, Haruma K, Oh-E H, et al. Conditions of curability after endoscopic resection for colorectal carcinoma with submucosally massive invasion. Oncol Rep 2000;7:783-788.


62. Choi YS, Kim WS, Hwang SW, et al. Clinical outcomes of submucosal colorectal cancer diagnosed after endoscopic resection: a focus on the need for surgery. Intest Res 2020;18:96-106.




63. Suh JH, Han KS, Kim BC, et al. Predictors for lymph node metastasis in T1 colorectal cancer. Endoscopy 2012;44:590-595.


64. Yasue C, Chino A, Takamatsu M, et al. Pathological risk factors and predictive endoscopic factors for lymph node metastasis of T1 colorectal cancer: a single-center study of 846 lesions. J Gastroenterol 2019;54:708-717.



65. Lee YJ, Huh JW, Shin JK, et al. Risk factors for lymph node metastasis in early colon cancer. Int J Colorectal Dis 2020;35:1607-1613.



66. Ichimasa K, Kudo SE, Mori Y, et al. Artificial intelligence may help in predicting the need for additional surgery after endoscopic resection of T1 colorectal cancer. Endoscopy 2018;50:230-240.


67. Kudo SE, Ichimasa K, Villard B, et al. Artificial intelligence system to determine risk of T1 colorectal cancer metastasis to lymph node. Gastroenterology 2021;160:1075-1084.


68. Luo X, Wang J, Han Z, et al. Artificial intelligence-enhanced white-light colonoscopy with attention guidance predicts colorectal cancer invasion depth. Gastrointest Endosc 2021;94:627-638.


69. Ozawa T, Kandimalla R, Gao F, et al. A microRNA signature associated with metastasis of T1 colorectal cancers to lymph nodes. Gastroenterology 2018;154:844-848.



- TOOLS








