 |
 |
- Search
| Intest Res > Volume 21(2); 2023 > Article |
|
Abstract
Background/Aims
Methods
Results
ADDITIONAL INFORMATION
Funding Source
This study was supported by the Indian Council of Medical Research (ICMR)-Center for Advanced Research in Intestinal Diseases (Ahuja V).
Author Contributions
Conceptualization: Kedia S, Ahuja V. Data curation: Sachdeva K, Singh MK, Verma M. Formal analysis: Sachdeva K, Singh MK. Funding acquisition: Ahuja V. Methodology: Sachdeva K, Kedia S, Ahuja V. Project administration: Kedia S, Ahuja V. Resources: Ahuja V. Software: Sachdeva K, Singh MK. Supervision: Makharia G, Kedia S, Ahuja V. Validation: Kedia S, Ahuja V. Visualization: Kedia S, Ahuja V. Writing - original draft: Sachdeva K, Kedia S, Ahuja V. Writing - review & editing: Kumar P, Kante B, Vuyyuru SK, Mohta S, Ranjan MK, Makharia G, Kedia S, Ahuja V. Approval of final manuscript: all authors.
Fig. 1.
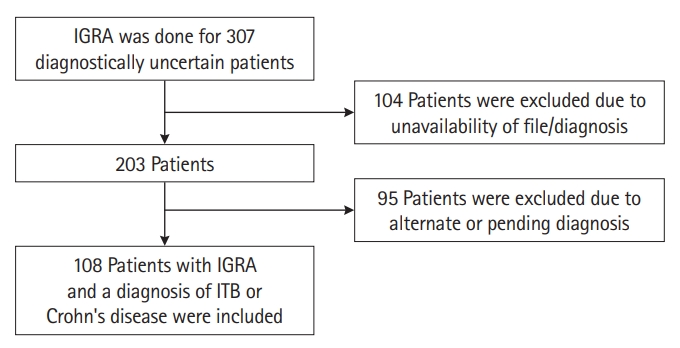
Fig. 2.

Fig. 3.
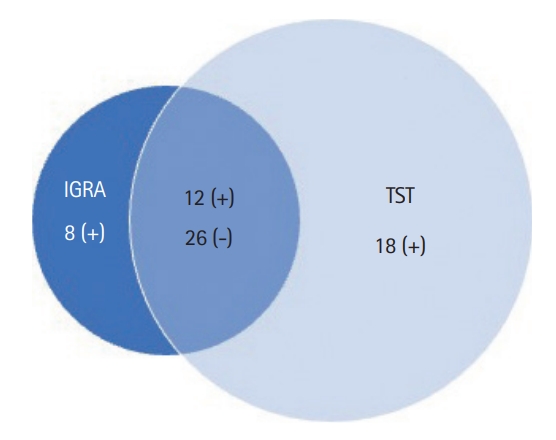
Table 1.
| Characteristics | Crohn’s disease (n = 49) | Intestinal tuberculosis (n = 59)b | P-value |
|---|---|---|---|
| Sex | 0.800 | ||
| Male (n = 65) | 30 (61.2) | 35 (59.3) | |
| Female (n = 43) | 19 (38.8) | 24 (40.7) | |
| Age (yr), mean ± SD | 37.8 ± 14.0 | 32.6 ± 13.1 | 0.050 |
| Duration of illness (yr) | < 0.001c | ||
| Mean | 5.1 | 2.1 | |
| Median (range) | 4 yr (3 mo-25 yr) | 1 yr (4 wk-20 yr) | |
| Clinical features | |||
| Diarrhea | 18 (36.7) | 15 (25.4) | 0.200 |
| Blood in stool | 18 (36.7) | 8 (13.6) | 0.005c |
| Abdominal pain | 38 (77.6) | 58 (98.3) | 0.001c |
| Intestinal obstruction | 11 (22.4) | 27 (45.8) | 0.012c |
| Weight loss | 32 (65.3) | 49 (83.0) | 0.030c |
| Hemoglobin (g/dL), mean ± SD | 10.1 ± 2.5 | 11.3 ± 2.5 | 0.010c |
| Perianal lesions | 7 (14.3) | 1 (1.6) | 0.020c |
| Endoscopy sitea | |||
| Left colon | 13 (26.6) | 4 (8.5) | 0.020c |
| Transverse colon | 7 (14.3) | 6 (12.8) | 0.800 |
| Ascending colon | 11 (22.4) | 11 (23.4) | 0.911 |
| Cecum | 10 (20.4) | 9 (19.1) | 0.876 |
| Terminal ileum | 19 (38.8) | 16 (34.0) | 0.630 |
| Endoscopy featuresa | |||
| Aphthous or serpiginous ulcers | 5 (10.2) | 0 | 0.010c |
| Transverse ulcers | 6 (12.2) | 9 (19.1) | 0.351 |
| Longitudinal ulcers | 7 (14.3) | 0 | 0.003c |
| Nodularity | 8 (16.3) | 4 (8.5) | 0.247 |
| Stricture | 8 (16.3) | 13 (27.6) | 0.179 |
| CT sitea | |||
| Left colon | 8 (16.3) | 1 (1.6) | 0.006c |
| Transverse colon | 5 (10.2) | 4 (6.8) | 0.520 |
| Ascending colon | 3 (6.1) | 8 (13.6) | 0.200 |
| Cecum | 6 (12.2) | 15 (25.4) | 0.080 |
| Terminal ileum | 26 (53.0) | 29 (49.1) | 0.670 |
| CT featuresa | |||
| Circumferential wall thickening | 25 (51.0) | 32 (54.2) | 0.670 |
| Long segment involvement | 9 (18.3) | 7 (11.9) | 0.340 |
| Asymmetric thickening | 10 (20.4) | 19 (32.2) | 0.170 |
| Stricture | 6 (12.2) | 6 (10.2) | 0.730 |
Table 2.
|
Crohn’s disease (n = 49) |
Intestinal tuberculosis (n = 59)b |
||||||
|---|---|---|---|---|---|---|---|
| IGRA+ (n=12) | IGRA- (n=37) | P-value | IGRA+ (n = 24)b | IGRA- (n = 35)b | P-value | ||
| Past history of TB | 6 (50.0) | 13 (35.1) | 0.50 | 4 (16.7) | 13 (37.1) | 0.14 | |
| TST (n = 64) | 4/6 (66.7) | 4/24 (16.7) | 0.03c | 8/14 (57.1) | 14/20 (70.0) | 0.48 | |
| Clinical features | |||||||
| Diarrhea | 3 (25.0) | 15 (40.5) | 0.50 | 7 (29.2) | 8 (22.9) | 0.76 | |
| Blood in stool | 4 (33.3) | 14 (37.8) | 0.99 | 2 (8.3) | 6 (17.1) | 0.45 | |
| Abdominal pain | 9 (75.0) | 29 (78.4) | 0.99 | 24 (100) | 34 (97.1) | 0.99 | |
| Intestinal obstruction | 6 (50.0) | 5 (13.5) | 0.02c | 7 (29.2) | 20 (57.2) | 0.06 | |
| Weight loss | 6 (50.0) | 26 (70.3) | 0.30 | 19 (79.2) | 30 (85.7) | 0.75 | |
| Hemoglobin (g/dL) | 10.74 ± 2.66 | 9.77 ± 2.39 | 0.24 | 11.29 ± 2.53 | 11.24 ± 2.56 | 0.94 | |
| Lymphocytes (%) | 26.04 ± 9.19 | 23.92 ± 12.85 | 0.50 | 25.82 ± 8.26 | 24.27 ± 8.17 | 0.65 | |
| Lymphocytes (absolute) | 1,858.27 ± 817.20 | 1,961.16 ± 1,239.40 | 0.80 | 1,837.62 ± 529.50 | 1,794.22 ± 585.60 | 0.79 | |
| Endoscopy sitea | |||||||
| Left colon | 1 (8.3) | 12 (32.4) | 0.14 | 1 (6.6) | 3 (9.3) | 0.76 | |
| Transverse colon | 1 (8.3) | 6 (16.3) | 0.66 | 2 (13.3) | 4 (12.5) | 0.94 | |
| Ascending colon | 1 (8.3) | 10 (27.0) | 0.25 | 3 (20.0) | 8 (25.0) | 0.71 | |
| Cecum | 1 (8.3) | 9 (24.3) | 0.41 | 4 (26.6) | 5 (15.6) | 0.37 | |
| Terminal ileum | 8 (66.7) | 11 (29.7) | 0.04c | 7 (46.6) | 9 (28.1) | 0.21 | |
| Endoscopy featuresa | |||||||
| Aphthous or serpiginous ulcers | 0 | 5 (13.5) | 0.31 | 0 | 0 | ||
| Transverse ulcers | 1 (8.3) | 5 (13.5) | 0.99 | 3 (20.0) | 6 (18.7) | 0.92 | |
| Longitudinal ulcers | 2 (16.7) | 5 (13.5) | 0.99 | 0 | 0 | ||
| Nodularity | 2 (16.7) | 6 (16.2) | 0.99 | 2 (13.3) | 2 (6.2) | 0.42 | |
| Stricture | 2 (16.7) | 6 (16.2) | 0.99 | 3 (20.0) | 10 (31.2) | 0.42 | |
| CT sitea | |||||||
| Left colon | 0 | 8 (21.6) | 0.17 | 0 | 1 (2.8) | 0.99 | |
| Transverse colon | 0 | 5 (13.5) | 0.31 | 1 (4.2) | 3 (8.5) | 0.63 | |
| Ascending colon | 1 (8.3) | 2 (5.4) | 0.99 | 2 (8.3) | 6 (17.1) | 0.45 | |
| Cecum | 1 (8.3) | 5 (13.5) | 0.99 | 6 (25.0) | 9 (25.7) | 0.99 | |
| Terminal ileum | 10 (83.3) | 16 (43.2) | 0.02 | 11 (45.8) | 18 (51.4) | 0.79 | |
| CT featuresa | |||||||
| Circumferential wall thickening | 3 (25.0) | 22 (59.5) | 0.05 | 11 (45.8) | 21 (60.0) | 0.28 | |
| Long segment involvement | 1 (8.3) | 8 (21.6) | 0.42 | 0 | 7 (20.0) | 0.03 | |
| Asymmetric thickening | 4 (33.3) | 6 (16.2) | 0.23 | 9 (37.5) | 10 (28.6) | 0.57 | |
| Stricture | 3 (25.0) | 3 (8.1) | 0.15 | 1 (4.16) | 5 (14.3) | 0.38 | |
a The frequencies for Endoscopy and CT findings do not add up due to few patients having normal findings and few having overlapping site/features.
Table 3.
| IGRA | Intestinal tuberculosis (n = 59) | Crohn’s disease (n = 49) | Total |
|---|---|---|---|
| Positive | 24 | 12 | 36 |
| Negative | 35 | 37 | 72 |
| Total | 59 | 49 | 108 |
Table 4.
| TST | Intestinal tuberculosis (n = 59) | Crohn’s disease (n = 49) | Total |
|---|---|---|---|
| Positive | 22 | 8 | 30 |
| Negative | 12 | 22 | 34 |
| Total | 34 | 30 | 64 |








