Korean clinical practice guidelines on biologics and small molecules for moderate-to-severe ulcerative colitis
Article information
Abstract
Ulcerative colitis (UC), a relapsing-remitting chronic inflammatory bowel disease (IBD), has a variable natural course but potentially severe disease course. Since the development of anti-tumor necrosis factor (TNF) agents has changed the natural disease course of moderate-to-severe UC, therapeutic options for patients who failed conventional treatments are expanding rapidly. IBD clinical trials have demonstrated the potential efficacy and safety of novel biologics such as anti-integrin α4β7 and anti-interleukin-12/23 monoclonal antibodies and small molecules such as a Janus kinase inhibitor. Anti-TNF biosimilars also have been approved and are widely used in IBD patients. Wise drug choices should be made considering evidence-based efficacy and safety. However, the best position of these drugs remains several questions, with limited data from direct comparative trials. In addition, there are still concerns to be elucidated on the effect of therapeutic drug monitoring and combination therapy with immunomodulators. The appropriate treatment regimens in acute severe UC and the risk of perioperative use of biologics are unclear. As novel biologics and small molecules have been approved in Korea, we present the Korean guidelines for medical management of adult outpatients with moderate-to-severe UC and adult hospitalized patients with acute severe UC, focusing on biologics and small molecules.
INTRODUCTION
Ulcerative colitis (UC), a relapsing-remitting chronic inflammatory bowel disease (IBD), has a variable natural course but potentially severe disease course. The median age at UC diagnosis was 36 years in a Korean population-based study [1], and 33–39 years in Western population-based studies [2,3]. The incidence of UC has increased in Korea over the past 3 decades, but remains lower than that among Westerners. Its mean annual incidence in Korea increased from 0.29/100,000 inhabitants in 1986–1990 to 5.82/100,000 inhabitants in 2011–2015 [1]. Although the majority of patients with UC have a mild-to-moderate disease course, 10%–15% of patients may experience an aggressive course, about a half of patients may require hospitalization for severe disease activity, and more than 80% of patients experience relapse [4,5]. The cumulative risks of colectomy at 1, 5, and 10 years after diagnosis of UC were 4.9%, 11.6%, and 15.6%, respectively, in Western population-based studies [6], but were only 1%, 1.9%, and 2.2%, respectively, in a Korean population-based study [7].
The risk of surgery for UC has decreased in the era of biologics. The development of anti-tumor necrosis factor (TNF) agents, including infliximab, adalimumab, and golimumab, has changed UC treatment. These agents have improved patients’ quality of life and altered the natural course of UC [8,9]. However, as many as one-third of IBD patients do not initially respond to anti-TNF agents, while another one-third lose their response during maintenance therapy [10-12]. Furthermore, although anti-TNF agents have acceptable safety profiles in general, there are concerns regarding opportunistic infections and malignancies [13,14]. Therefore, drugs that affect pathways involved in the pathogenesis of UC should be developed. As the pathological mechanisms of IBD have been further elucidated, IBD clinical trials have demonstrated the potential efficacy and safety of novel drugs. Korean guidelines for management of UC were published in 2012 and revised in 2017 by the IBD Research Group of the Korean Association for the Study of Intestinal Diseases (KASID) [15,16]. The revised guidelines provided 4 treatment recommendations about biologics, including antiTNF agents to induce and maintain remission, dose escalation and monitoring of anti-TNF agents, and an anti-integrin α4β7 monoclonal antibody (vedolizumab) to maintain remission [16]. Since then, novel biologics such as anti-integrin α4β7 and anti-interleukin-12/23 monoclonal antibodies (ustekinumab) and small molecules such as a Janus kinase (JAK) inhibitor (tofacitinib) have expanded indications and been approved in Korea [17].
Therapeutic options for UC are expanding rapidly. New biologics and small molecules may help to overcome the limitations of anti-TNF agents and be used to treat patients in whom treatment with anti-TNF agents has failed. Therefore, new UC management guidelines are needed. Here, we present the third guidelines for medical management of adult outpatients with moderate-to-severe UC and adult hospitalized patients with acute severe UC (ASUC), focusing on biologics and small molecules. These guidelines do not provide an absolute therapeutic approach, but may help physicians to choose evidence-based treatment options to manage moderate-to-severe UC patients.
METHOD
1. Planning and Directions
In October 2020, the IBD Research Group of the KASID agreed to develop the third guidelines for UC management in order to update those that were published in 2017. These second revised guidelines focus on biologics and new small molecules, such as infliximab, adalimumab, golimumab, vedolizumab, ustekinumab, and tofacitinib, which have been approved for UC management in Korea. To create the guidelines, the KASID selected a panel of 13 IBD experts including the chairman Choi CH, 11 medical physicians, and 1 surgeon supported by 1 methodologist (Miyoung Choi; National Evidence-based Healthcare Collaborating Agency). Radiology and pathology experts were excluded because this revision focuses on treatment with biologics and small molecules. None of the authors of the guideline development working group have a conflict of interest.
2. Development Process
1) Key Questions and Development Methods
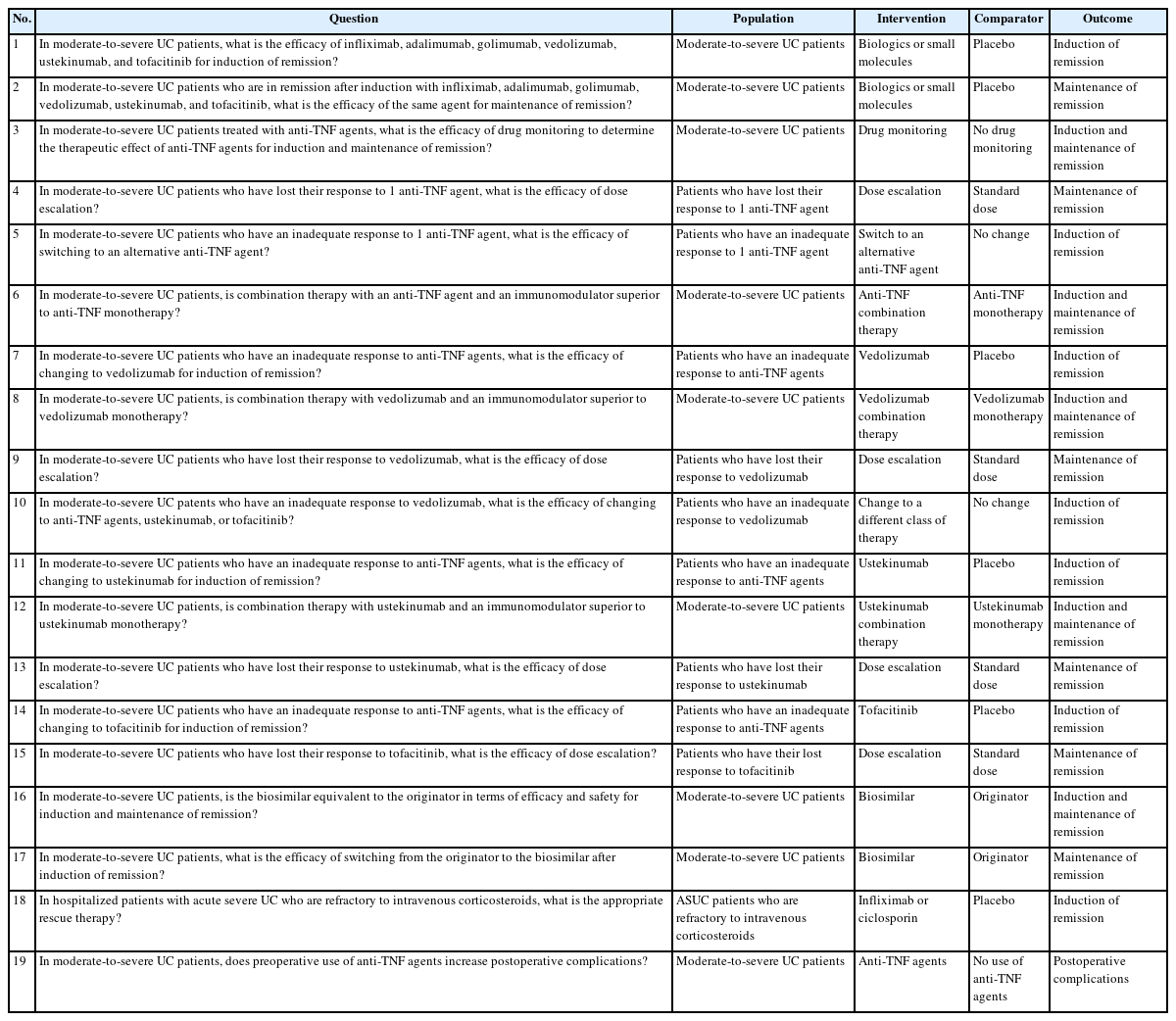
The key clinical questions were selected from those suggested about the use of biologics and small molecules during the treatment of IBD patients. The committee members formulated 19 clinically important questions regarding biologics and small molecules using the PICO (population, intervention, comparator, patient-important outcomes) format. The initial key questions are summarized in Table 1. As the committee determined the scope of the new guidelines, it performed a systematic search and reviewed the existing guidelines to assess the relevant evidence in order to address the clinical questions. Relevant guidelines have already been developed in various countries using high quality, internationally accepted, and appropriate methodology. The guideline development committee decided to select the “adaptation” method among the 3 guideline development methods (de novo, adaptation, and hybrid).
2) Search Strategy and Selection for Reference Guidelines
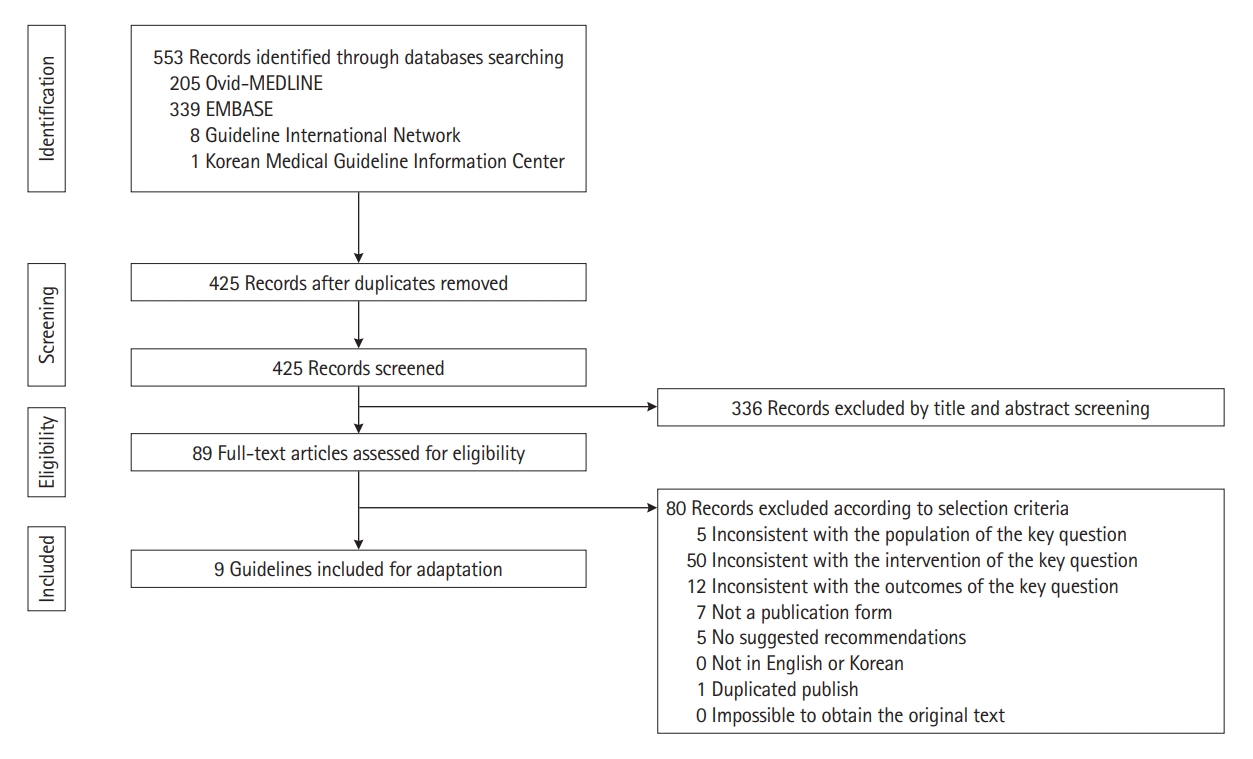
After creating the key questions, we searched the relevant preceding guidelines for adaptation according to the search strategy. We identified 553 articles published between January 1, 2015, and October 1, 2020, by searching MEDLINE, EMBASE, the Guidelines International Network, and Korean Medical Guideline Information Center using keywords including “colitis, ulcerative” and “inflammatory bowel disease.” The titles and abstracts of the searched literature were screened, and full-text articles were assessed for eligibility. Based on the pre-defined exclusion criteria, secondary screening was performed after reviewing the original text of the selected articles. This was performed by 2 independent experts of the committee. If there was a disagreement between them, an agreement was reached after discussion. Fig. 1 shows a flowchart of the screening. Nine guidelines were assessed for suitability for adaptation using Korean Appraisal of Guidelines for Research and Evaluation (K-AGREE) II by 2 independent committee members. The K-AGREE evaluation tool consists of 6 domains, namely, scope and purpose, stakeholder involvement, rigor of development, clarity of presentation, applicability, and editorial independence [18]. Guidelines for which the subscore in the “rigor of development” area was less than 50% or evaluated as “not recommended” based on the total score were excluded. Finally, we identified 7 evidence-based, peer-reviewed, and national or international guidelines (Table 2) [16,19-24].
3) Assessment of Risk of Bias
After selecting the reference guidelines for adaptation, the guideline development committee searched for studies reported after the 7 reference guidelines via PubMed that could be included as evidence for our guidelines according to the key questions. Two independent committee members separately assessed the quality of the individual studies cited in the selected guidelines and the latest studies for each PICO question using RoB 1.0 (randomized controlled trials, RCTs) and RoBANS 2.0 (obervational studies) (Supplementary Tables 1-4). During this step, 8 key questions were excluded due to a lack of direct evidence or knowledge gap. Finally, it was decided to make recommendations that matched 11 key questions.
4) Quality of Evidence and Grade of Recommendation
The guideline development committee formulated statements for the clinical key questions according to the quality of evidence and grade of recommendation. The former was classified into 4 categories, from high to very low, according to the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) classification (Table 3) [25]. The level of the quality of evidence was classified from high to low confidence according to the design of the supporting studies for each key question. Subsequently, it was downrated if there was a risk of bias, imprecision, indirectness, inconsistency, and publication bias; and uprated if there were dose-response results, large effects, and no probable confounding that would No. decrease an exhibited effect or indicate a false effect.
The determinants of the strength of each recommendation were graded as “strong” or “conditional” by considering the quality of evidence, benefit-harm balance, values and preferences of patients, resources, and cost (Table 4). The direction of each recommendation was defined as “for” or “against.” Decisions about the quality of evidence and grade of recommendation were first made by 2 independent guideline development committee members, and finally revised and decided by the guideline developing working group through a general consensus meeting.
The draft was reviewed and approved by the external advisory committee, which was composed of 5 IBD experts who are members of the KASID. All comments were collected, reviewed, and addressed by the guideline committee. The final version of the third Korean guidelines for the management of UC was presented at the International Meeting on Intestinal Diseases in Conjunction with the Annual Congress of the Korean Association for the Study of Intestinal Diseases (IMKASID) 2021.
DISEASE ACTIVITY OF MODERATE-TO-SEVERE UC
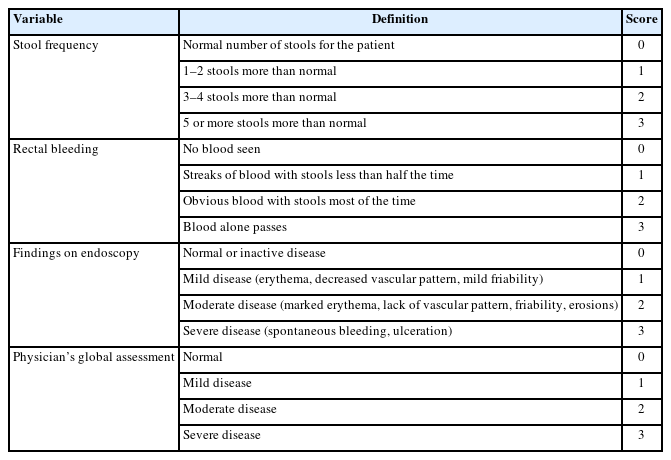
The extent, severity, and clinical features of UC determine the treatment options. These guidelines address the medical management of adult outpatients with moderate-to-severe UC and hospitalized patients with ASUC. After exclusion of patients with concomitant infections (e.g., Clostridioides difficile), for those with moderate-to-severe UC and who are corticosteroid-dependent or -refractory or who have severe endoscopic disease activity (large and/or deep ulcers), biologics or small molecules are indicated. Moderate-to-severe UC is defined according to the modified Truelove and Witts criteria (Table 5) [26,27] or Mayo score (Table 6) [28,29] in these guidelines. Hospitalized patients with the following modified Truelove and Witts criteria are defined as having ASUC in these guidelines: more than 6 bloody bowel movements per day and at least 1 marker of systemic toxicity, such as heart rate > 90/min, temperature > 37.8°C, hemoglobin level < 10.5 g/dL, erythrocyte sedimentation rate > 30 mm/hr, and C-reactive protein level > 30 mg/L, or a Mayo score of more than 10 points. The risk of in-hospital colectomy is very high for ASUC patients, especially those who have several systemic toxicity markers [21]. For these guidelines, clinical outcomes for decision-making were induction and maintenance of remission in adults outpatients with moderate-to-severe UC and the short-term colectomy risk (within 3 months of hospitalization) in hospitalized adults with ASUC.
MANAGEMENT OF MODERATE-TO-SEVERE UC
Conventional therapy for moderate-to-severe UC patients includes 5-aminosalicylic acid (5-ASA), immunomodulators, and corticosteroids. A meta-analysis of 5 RCTs reported that corticosteroids had a superior efficacy relative to placebo for induction of remission (relative risk [RR], 0.65; 95% confidence interval [CI], 0.45–0.93) [30]. The optimal dosage of corticosteroid is mostly 40–60 mg/day oral prednisolone because there is no additional dose-response effect with a higher dosage [31]. Approximately 15%–25% of UC patients need to be hospitalized due to an acute severe flare-up [32]. ASUC patients with symptoms of systemic toxicity, which is a medical emergency, require high-dose intravenous (IV) corticosteroids such as 40–60 mg methylprednisolone or 100 mg hydrocortisone every 6 hours daily [21]. The type of injection (bolus or continuous) does not influence the effect [33].
The development of anti-TNF agents has changed UC treatment. These agents have been a gamechanger in UC management, improved quality of life, and changed the disease’s natural course [8,9]. However, as many as one-third of patients may not respond to anti-TNF agents, while another 10%–15% may lose their response each year [34,35]. The low therapeutic drug level may be owing to increased drug clearance because the inflammatory burden is increased, loss of protein across the inflamed and permeable mucosa, production of neutralizing antidrug antibodies (ADAs), or factors related to the patient including male sex and an elevated body mass index [36,37]. Therapeutic drug monitoring (TDM) would help to make decisions regarding treatment options in patients who fail to respond or lose their response to anti-TNF agents. In patients with a sufficient trough level of anti-TNF agents, changing to a drug that has a different mechanism-of-action is recommended over cycling with other anti-TNF-agents [20]. When the trough level of anti-TNF agents is low, dose escalation may be an option in cases with low ADA titers, while switching to another anti-TNF agent may be possible in cases with high ADA titers [20].
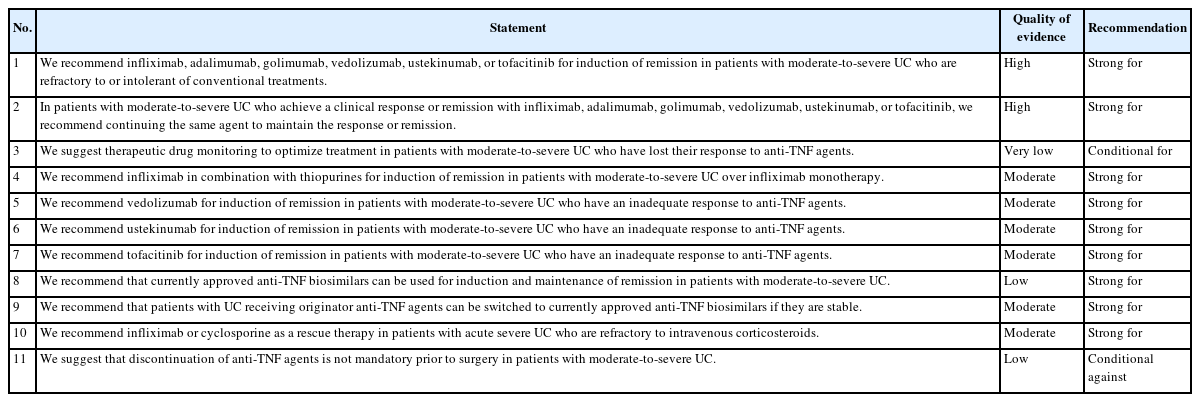
Novel biologics, including vedolizumab and ustekinumab, and small molecules, including tofacitinib, are approved for moderate-to-severe UC treatment in Korea (Fig. 2) [17]. Moreover, ozanimod (S1P receptor modulator) was approved by the U.S. Food and Drug Administration (FDA) [38], although it has not yet been approved in Korea. However, it is difficult to choose the best drugs for management of moderate-to-severe UC because head-to-head comparative trials are lacking. Data concerning comparative efficacy in terms of endoscopic and clinical outcomes will help decide which therapeutic agents to choose. Nevertheless, the general principle when choosing therapeutic options for UC is striking a balance between evidence-based efficacy and safety profiles. Table 7 summarizes the recommendations, quality of evidence, and strength of recommendations of the third Korean guidelines for the management of UC.
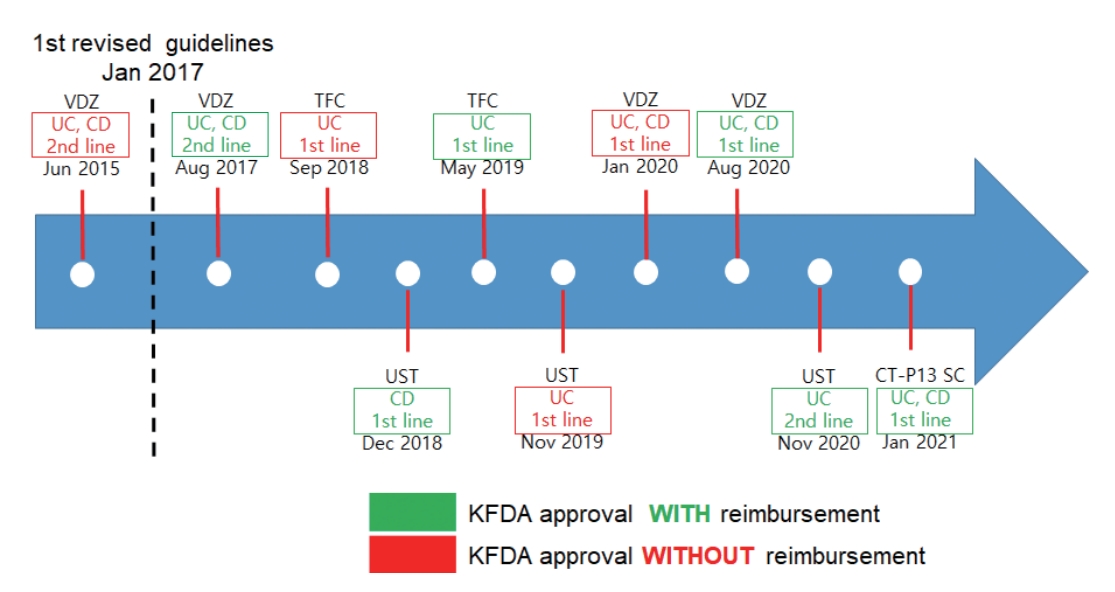
Korean Food and Drug Administration (KFDA) approval and reimbursement guidelines for novel biologics and small molecules in patients with moderate-to-severe ulcerative colitis (UC). VDZ, vedolizumab; CD, Crohn’s disease; TFC, tofacitinib; UST, ustekinumab; SC, subcutaneous.
SAFETY CONCERNS
Click and Regueiro39 proposed a safety pyramid of biologics and small molecules. The order of safety is as follows: vedolizumab, ustekinumab, anti-TNF monotherapy, thiopurines or tofacitinib, and anti-TNF combination therapy.
1. Anti-TNF Agents
Opportunistic infections with both bacterial and fungal etiologies are associated with the use of anti-TNF agents. In comparison with the general population, the risk of active tuberculosis is increased by 2- to 8-fold in patients treated with anti-TNF agents [40]. Tuberculosis is reactivated in 0.05% of patients taking anti-TNF agents [39], and its incidence increases to 1%–2% in endemic areas [41]. According to analysis by the U.S. FDA Adverse Event Reporting System, the risk of tuberculosis infection was higher in patients receiving anti-TNF monotherapy (odds ratio [OR], 8.52; 95% CI, 1.96–37.01; P<0.001), anti-TNF combination therapy (OR, 25.27; 95% CI, 5.66–112.72; P<0.001), and anti-TNF agents plus systemic corticosteroids (OR, 8.46; 95% CI, 1.88–38.21; P=0.001) than in patients receiving 5-ASA [42]. In a large French population cohort, the incidence of lymphoma among 189,289 IBD patients was 0.54/1,000 person-years (95% CI, 0.41–0.67) for patients receiving only thiopurines and 0.95/1,000 person-years (95% CI, 0.45–1.45) for patients receiving thiopurines plus anti-TNF agents. The risk of lymphoma increases with combination treatment and therefore patients receiving anti-TNF agents and thiopurines must be closely observed [43].
2. Vedolizumab (Anti-Integrin α4β7 Monoclonal Antibody)
Vedolizumab is relatively safe and has minimal systemic immunosuppressive action due to its gut specificity. In the GEMINI study, the efficacy and safety of vedolizumab were comparable in all age groups [44]. In addition, vedolizumab did not increase the risk of any infection, serious infection, or malignancies in more than 2,800 patients over 5 years compared with the prognosis of general IBD patients [45]. Therefore, vedolizumab can be a good alternative for UC patients who have a high risk of infections or malignancies.
3. Ustekinumab (Anti-Interleukin-12/23 Monoclonal Antibody)
In the UNIFI study, ustekinumab did not increase the risk of serious adverse events or serious infections at any age [46]. In the IM-UNITI long-term extension study of Crohn’s disease (CD), ustekinumab did not increase the risk of adverse events or serious adverse events or infections up to week 96 compared with placebo, except in 1 patient with tuberculosis in an endemic area [47]. Long-term data from Psoriasis Longitudinal Assessment and Registry (PSOLAR) showed that ustekinumab was safe and that serious infections and malignancies were rare [48,49]. Therefore, ustekinumab can be a good alternative for UC patients who have a high risk of infections or malignancies.
4. Tofacitinib (JAK Inhibitor)
The safety of tofacitinib has mostly been studied in rheumatologic diseases. A meta-analysis of 5 RCTs reported that treatment with 5 or 10 mg tofacitinib twice daily had similar efficacies in older and young patients, but side effects were more common in older patients [50]. Elderly patients who received 5 or 10 mg tofacitinib twice daily had a higher incidence of serious infections (incidence rate [IR], 7.6 vs. 4.7 vs. 0 per 100 person-years), opportunistic infections (IR, 0.76 vs. 2.35 vs. 0 per 100 person-years), and herpes zoster (IR, 3.1 vs. 5.5 vs. 0 per 100 person-years) than elderly patients who received placebo [50]. Several clinical studies suggested a relationship between JAK inhibitors and venous thromboembolism [51]. In subsequent post-marketing safety studies, among patients with 1 or more cardiovascular disease risk factors who were older than 50 years, the risk of venous thromboembolism was higher in patients receiving 10 mg twice daily than in patients receiving 5 mg twice daily [52]. Therefore, the U.S. FDA inserted a warning box for a dosage of 10 mg twice daily in February 2019, and the European Medicines Agency advised caution [53,54]. A recent, large, randomized safety trial reported that tofacitinib increased the risk of severe heart-related events, including stroke and heart attack, blood clots, cancer, and death in rheumatoid arthritis patients compared with anti-TNF agents [55]. In September 2021, the FDA stipulated that warnings should be provided about the increased risks of these adverse events associated with JAK inhibitors used to treat certain chronic inflammatory conditions [56]. The FDA now suggests that tofacitinib is reserved for patients who are intolerant of or respond inadequately to 1 or more anti-TNF agents [56]. However, tofacitinib was approved as a first-line drug in Korea in September 2018. Therefore, the benefits and risks for each patient should be considered when using tofacitinib according to these guidelines.
RECOMMENDATION
Statement 1
We recommend infliximab, adalimumab, golimumab, vedolizumab, ustekinumab, or tofacitinib for induction of remission in patients with moderate-to-severe UC who are refractory to or intolerant of conventional treatments (Strong recommendation, High quality of evidence).
Thirteen RCTs compared the efficacy of induction therapy using anti-TNF agents, vedolizumab, ustekinumab, or tofacitinib with placebo in moderate-to-severe UC patients [44,46,57-65]. In the ACT-1 and ACT-2 trials, in which 45.9% of patients (334/728) did not respond to immunomodulators, a significantly higher clinical response rate was achieved at week 8 using induction therapy with 5 or 10 mg/kg infliximab than using placebo (33.2% vs. 66.9% vs. 15.3%) [57]. In the ULTRA-1 and ULTRA-2 trials, a significantly higher clinical remission rate was achieved using induction therapy with adalimumab (160 mg at week 0, 80 mg at week 2, and 40 mg at weeks 4 and 6) than using placebo (18.5% vs. 9.2% in ULTRA-1 and 16.5% vs. 9.3% in ULTRA-2) [60,61]. In the PERSUIT trial, in which 32.4% of patients (345/1,065) did not tolerate immunomodulators, the clinical response rate at week 6 was significantly higher in patients treated with 200 mg/100 mg golimumab (51.0%) or 400 mg/200 mg golimumab (54.9%) than in patients treated with placebo (30.3%) (P<0.0001) [63]. In the GEMINI-1 trial, the clinical response rate at week 6 was significantly higher in the group treated with 300 mg vedolizumab at weeks 0 and 2 than in placebo-treated patients (47.1% vs. 25.5%, P<0.001) [44]. In the UNIFI trial, in which 28.2% of patients (271/961) had a history of immunomodulator or biologic failure, a significantly higher clinical remission rate was achieved at week 8 using induction therapy with 6 mg/kg or 130 mg ustekinumab than using placebo (15.5% or 15.6% vs. 5.3%) [46]. In the OCTAVE-1 and OCTAVE-2 trials, in which 71.9% of patients (819/1,139) had a history of immunomodulator or biologic failure, patients treated with 10 mg tofacitinib had a significantly higher clinical remission rate at week 8 than those treated with placebo (P<0.001) [65]. In patients who undergo induction therapy with biologics and small molecules, the response should be assessed after weeks 6–16 of initiating therapy according to the Korean reimbursement criteria for each drug used as follows: response to infliximab, adalimumab, and golimumab after 6, 8, 10 weeks of the first dose, respectively; response to vedolizumab, ustekinumab, and tofacitinib after week 6, at weeks 16–20, and at week 16 of the first dose, respectively.
All approved agents showed efficacy in terms of induction of remission in moderate-to-severe UC patients, and only 1 head-to-head trial has compared the currently available biologic therapies. Therefore, the drug should be chosen by considering various factors, such as the underlying disease, presence of poor prognostic factors, presence of extraintestinal manifestations (EIMs), possibility of pregnancy, adherence and preference of patients, reimbursement policies and cost, and safety profiles of drugs. Among the approved biologics and small molecules, anti-TNF agents and ustekinumab are preferred to treat EIMs of UC. In a systematic review of 9 interventional studies including 2 RCTs, anti-TNF agents were effective for treating EIMs of IBD including musculoskeletal, cutaneous, and ocular manifestations [66]. A recently published systematic review showed that ustekinumab is also beneficial for many EIMs of IBD including rheumatologic manifestations and cutaneous manifestations such as psoriasis, pyoderma gangrenosum, and erythema nodosum [67]. Data regarding the effects of vedolizumab and tofacitinib on EIMs in UC patients are lacking, and more data are needed to confirm their effectiveness in this context.
The VARSITY trial, a recently published head-to-head trial, compared vedolizumab and adalimumab for treatment of moderate-to-severe UC. The clinical remission rate at week 52 was significantly higher in the vedolizumab-treated group than in the adalimumab-treated group among biologic-naïve patients (34.2% vs. 24.3%; difference 9.9%; 95% CI, 2.8–17.1) [68]. A network meta-analysis comparing infliximab, adalimumab, golimumab, vedolizumab, and tofacitinib for treatment of moderate-to-severe UC (including 15 RCTs) ranked infliximab highest in terms of induction of clinical remission in biologic-naïve patients (OR, 4.07; 95% CI, 2.67–6.21; surface under the cumulative ranking [SUCRA], 0.95) and endoscopic improvement (SUCRA, 0.95) [69]. Vedolizumab was ranked relatively higher for induction of clinical remission (SUCRA, 0.63) and endoscopic improvement (SUCRA, 0.76) in biologic-naïve patients with moderate-to-severe UC [69]. Therefore, infliximab or vedolizumab may be more suggested than adalimumab for induction of remission in moderate-to-severe UC patients who are naïve to biologics, although the quality of evidence is limited.
“Accelerated step-up” therapy with biologics or small molecules could be considered in moderate-to-severe UC patients with poor prognostic factors. Given that uncontrolled UC is associated with elevated colectomy and hospitalization risks, early use of biologics or small molecules may help moderateto-severe UC patients achieve remission. However, data comparing “accelerated step-up” and “conventional step-up” therapies in moderate-to-severe UC patients are limited [70]. Therefore, treatment should be based on the risk and benefit assessment, considering the side effects and costs of drugs.
Statement 2
In patients with moderate-to-severe UC who achieve a clinical response or remission with infliximab, adalimumab, golimumab, vedolizumab, ustekinumab, or tofacitinib, we recommend continuing the same agent to maintain the response or remission (Strong recommendation, High quality of evidence).
In 16 RCTs, anti-TNF agents, vedolizumab, ustekinumab, and tofacitinib were superior to placebo for maintenance of remission (infliximab: RR, 2.25; 95% CI, 1.67–3.05; adalimumab: RR, 2.28; 95% CI, 1.52–3.42; golimumab: RR, 1.88; 95% CI, 1.32–2.68; vedolizumab: RR, 2.31; 95% CI, 1.63–3.28; tofacitinib: RR, 3.09; 95% CI, 1.99–4.79; ustekinumab: RR, 1.83; 95% CI, 1.33–2.49) [71]. Maintenance therapy with these medications had low rates of serious adverse events, and these rates did not significantly differ from that observed with placebo. A dosage of 5 mg twice daily is recommended for long-term maintenance with tofacitinib. A higher dosage should only be used in patients who lose their response at this dosage because the risk of venous thromboembolism might be increased in patients on a higher dosage of tofacitinib [52].
It is unclear if biologic monotherapy is favored over thiopurine monotherapy to maintain remission after induction with corticosteroids and biologics [19]. The efficacies of biologics and thiopurines for maintenance of remission have not been compared in a clinical trial because UC-SUCCESS was terminated early [72]. 5-ASA can be withdrawn for induction and maintenance of remission in moderate-to-severe UC outpatients in whom it has failed and who have been switched to a biologic and/or immunomodulators or tofacitinib [73,74]. A meta-analysis showed that the remission rates of anti-TNF agent- or tofacitinib-treated patients with or without concomitant 5-ASA did not differ (RR, 0.92; 95% CI, 0.78–1.09) [71].
Episodic injection of anti-TNF agents is not recommended because it can lead to the generation of ADAs. The incidence of ADAs was 60% in patients receiving infliximab episodically, but only 6%–25% in those receiving scheduled infusions [75-77]. ADAs to infliximab are associated with reduced serum levels of infliximab, infusion reactions, and, in the majority of studies, loss of response [36,78-80].
Statement 3
We suggest TDM to optimize treatment in patients with moderate-to-severe UC who have lost their response to anti-TNF agents (Conditional recommendation, Very low quality of evidence).
TDM has been increasingly adopted as a potential strategy to optimize anti-TNF agent therapy. One small RCT evaluated the effect of reactive TDM in patients with IBD. In this RCT, 69 CD patients with recurred symptoms on maintenance therapy with infliximab were randomly assigned to TDM-guided treatment changes or empiric dose escalation [81]. Although the cost of TDM-guided treatment was significantly lower than that of empiric dose escalation, attainment of remission at week 12 was similar in the 2 groups. However, these results should be carefully interpreted because the trough level of infliximab (≥ 0.5 µg/mL) classified as therapeutic in this study is considerably lower than that of infliximab (≥ 5 µg/mL) which is widely used in recent clinical practice.
Reactive TDM may help to guide changes in treatment for patients who lose their response. Observational studies showed a beneficial effect of reactive TDM over empirical dose escalation or switching to another class in patients who lost their response to anti-TNF agents [82-85]. In a pooled analysis incorporating 2 studies, 45% (60/134) of patients responded to dose escalation.82,83 Among them, 82% (41/50) and 8% (2/24) of patients with a subtherapeutic trough level without ADAs and with high ADAs responded to dose escalation, respectively. Yanai et al. [84] reported similar results (55% of patients with loss of response and no/low ADAs vs. 15% of patients with loss of response and high ADAs). Patients who once responded to anti-TNF agents but have now lost this response could be managed based on their trough drug levels and antibodies. For patients with adequate serum levels of anti-TNF agents and higher levels of ADAs, a change to another class of drug is recommended rather than dose escalation or cycling with another anti-TNF agent [85]. Based on a network meta-analysis of 7 RCTs including 1,580 patients exposed to anti-TNF agents, ustekinumab or tofacitinib rather than adalimumab or vedolizumab is suggested for primary non-responders to infliximab [69]. However, this network meta-analysis has a limited quality of evidence because it is not a direct comparative study and caution is required when interpretating its findings.
Evidence supporting routine proactive TDM is limited and the overall benefit remains uncertain. Although there has been no RCT that evaluates the role of proactive TDM for achieving induction of remission, 2 RCTs investigated the role of proactive TDM in the maintenance treatment of patients with IBD. In the TAXIT trial, all patients in whom the dose of infliximab was first optimized with 3–7 µg/mL as a target trough level were randomly assigned to the proactive TDM or non-TDM group [86]. Attainment of clinical remission was similar in the 2 groups at 1 year; however, there was a lower rate of disease flare-ups and a cost-saving effect due to dose reduction in patients with a supratherapeutic trough level in the proactive TDM group [86]. In the TAILORIX trial, dose intensification based on clinical symptoms and the serum infliximab level did not elicit any significant beneficial effect compared with dose intensification based on clinical symptoms only [87]. Based on these results, a recommendation regarding routine proactive TDM in moderate-to-severe UC patients receiving anti-TNF agents cannot be made. However, the importance of proactive TDM is gradually emerging and it is therefore expected to be used more actively in the future.
Statement 4
We recommend infliximab in combination with thiopurines for induction of remission in patients with moderate-to-severe UC over infliximab monotherapy (Strong recommendation, Moderate quality of evidence).
A randomized, double-blind, three-arm trial called the UCSUCCESS study investigated the efficacy of treatment with a combination of infliximab and thiopurine compared with infliximab or thiopurine monotherapy in 239 moderate-to-severe UC patients who were naïve to anti-TNF agents. Corticosteroid-free remission (39.7% vs. 22.1%, P=0.017), but not mucosal healing (62.9% vs. 54.6%, P=0.295), was more likely to be achieved at week 16 by patients on combination therapy than by patients on infliximab monotherapy [72]. This finding is attributed to the better pharmacokinetics of the biologics when added to immunomodulators (elevated trough level and less immunogenicity). This study also showed that corticosteroidfree remission was more effectively induced by combination therapy than by azathioprine monotherapy (39.7% vs. 23.7%, P=0.032). The UC-SUCCESS study was prematurely terminated before completion of the maintenance period and therefore did not provide information about maintenance of remission. A retrospective multicenter study incorporating 82 patients in remission on infliximab and azathioprine reported that fewer patients on combination therapy suffered clinical relapse than patients who stepped down to infliximab monotherapy (median follow-up, 22.3 months; P=0.049) [88]. This result suggests that combination therapy has a better clinical outcome than monotherapy during maintenance. On the other hand, the additive benefit of azathioprine and adalimumab is unclear [89-91]. Therefore, adalimumab may be an alternative when clinicians are considering anti-TNF agent use in patients who are intolerant of or suffer adverse events in response to immunomodulators.
Statement 5
We recommend vedolizumab for induction of remission in patients with moderate-to-severe UC who have an inadequate response to anti-TNF agents (Strong recommendation, Moderate quality of evidence).
The anti-integrin antibody vedolizumab is expected to be efficacious as a second-line treatment after failure of anti-TNF agents in moderate-to-severe UC patients because it has a different mechanism-of-action [16]. In the induction trial of GEMINI 1, vedolizumab-treated patients (n = 225) had higher clinical response, clinical remission, and mucosal healing rates than placebo-treated patients (n = 149) at week 6 (47.1% vs. 25.5%, 16.9% vs. 5.4%, and 40.9% vs. 24.8%, all P<0.001) [44]. Among patients receiving vedolizumab, 42.2% had been previously exposed to anti-TNF agents and 36.4% had at least 1 anti-TNF agent failure experience. For patients with a history of anti-TNF agent failure, the clinical response at week 6 was significantly higher among those treated with vedolizumab than among those treated with placebo (39.0% vs. 20.6%; 95% CI, 3.9–32.9; P=0.01). The maintenance trial also found that vedolizumab was superior to placebo in patients with a history of anti-TNF agent failure in terms of clinical remission. For patients with a history of anti-TNF agent failure, the clinical remission rate at week 52 was 37.2% and 35.0% among those who continued to receive vedolizumab every 8 or 4 weeks, respectively, but was only 5.3% among those who received placebo (all P<0.001). Post-hoc analysis of the GEMINI 1 trial revealed that patients who received 3 induction doses of vedolizumab at weeks 0, 2, and 6 were more likely to be in sustained clinical remission at weeks 14–54 that patients who received placebo. The results were consistent in patients who had a history of anti-TNF agent treatment. For patients with a history of anti-TNF agent failure, the percentage of patients in clinical remission, as assessed by a rectal bleeding subscore of 0, at weeks 14, 26, 38, and 52 was significantly higher among those treated with vedolizumab (50%–60%) than among those treated with placebo (20%) [92]. The GEMINI-1 study, including the post-hoc analysis, showed that vedolizumab is effective in moderate-to-severe UC patients in the short- and long-term, regardless of whether they have a history of anti-TNF agent exposure.
A recent phase 3 RCT of Japanese moderate-to-severe UC patients reported that the clinical remission rate at week 60 significantly differed between the vedolizumab- and placebo-treated groups (56.1% vs. 31.0%; adjusted OR, 2.88; 95% CI, 1.168–7.108), but the clinical response at week 10 did not. Subgroup analyses of patients with a history of anti-TNF agent use yielded similar findings. Among patients with a history of anti-TNF agent failure, the clinical response, clinical remission, and mucosal healing rates at week 10 did not differ between the subgroups, but the clinical remission and mucosal healing rates at week 60 were significantly higher in the vedolizumab-treated group than in the placebo-treated group (58.8% vs. 21.4%; adjusted OR, 37.4; 95% CI, 5.625–69.165; and 64.7% vs. 28.6%; adjusted OR, 36.1; 95% CI, 3.332–68.937) [64].
The first head-to-head trial comparing different biologics in IBD, the VARSITY trial, showed the superior efficacy of vedolizumab over adalimumab for moderate-to-severe UC [68]. However, the benefit was not observed in patients who had a history of anti-TNF agent therapy. Specifically, the clinical remission rate was 20.3% and 16% in the vedolizumab- and adalimumab-treated groups at week 52, respectively, among patients with a history of treatment with an anti-TNF agent other than adalimumab [68]. However, vedolizumab and ustekinumab or tofacitinib have not been compared in a randomized trial. A recent network meta-analysis including 7 RCTs with 1,580 patients revealed that second-line vedolizumab is less effective than ustekinumab or tofacitinib in terms of induction of remission in patients with a history of anti-TNF agent exposure (tofacitinib vs. vedolizumab: OR, 6.18; 95% CI, 1.00–38.00; ustekinumab vs. vedolizumab: OR, 5.99; 95% CI, 1.13–31.76) [69]. Confidence in the estimates of induction of clinical remission using vedolizumab as a second-line therapy over placebo was low in a direct meta-analysis (OR, 1.55; 95% CI, 0.58–4.16) [65]. However, the paucity and imprecision of the included studies and lack of dose intensification might attenuate the efficacy of vedolizumab [71].
The benefit of dose intensification of vedolizumab for both induction and maintenance has been suggested based on recent observational studies [93-96]. In GEMINI LTS, the response rate was 53.1% (17/32) at week 28 among the subset of patients who received dose intensification from 8 weeks interval to 4 weeks interval [97]. A systematic review and meta-analysis reported that the pooled IRs of loss of response were 39.8/100 person-years follow-up among UC patients [98]. The efficacy rate of vedolizumab intensification was 53.8% in secondary non-responders with CD and UC [98]. Regarding the efficacy of concomitant immunomodulators, the benefit of combination therapy over vedolizumab monotherapy is unclear. A recent meta-analysis evaluated the efficacy of combination therapies excluding anti-TNF agents. In subgroup analysis including 7 observational studies, there was no benefit of inclusion of an immunomodulator over monotherapy in patients receiving vedolizumab (OR, 0.92; 95% CI, 0.60–1.41; I2 = 21%) [99]. Although no trials have specifically compared vedolizumab monotherapy and combination therapy, this result is consistent with the low immunogenicity of vedolizumab [44].
Vedolizumab, a gut-selective biologic targeting α4β7 integrin, has a low risk of causing opportunistic infections. Less than 0.6% of patients were reported to have serious clostridial infection, tuberculosis, and sepsis [45]. In clinical trials including GEMINI 1, GEMINI 2, and GEMINI LTS, the incidence of tuberculosis was 0.1/100 person-years [100]. A network meta-analysis reported that the risk of infection was lowest for vedolizumab (SUCRA, 0.81) [69]. Thus, vedolizumab is potentially safe in terms of reactivation of latent tuberculosis or primary tuberculosis. Together with its efficacy, the favorable safety profile of vedolizumab makes it usable as a first- and second-line treatment in moderate-to-severe UC patients, especially in endemic regions such as Asia-Pacific countries [101].
Statement 6
We recommend ustekinumab for induction of remission in patients with moderate-to-severe UC who have an inadequate response to anti-TNF agents (Strong recommendation, Moderate quality of evidence).
The UNIFI trial investigated the effects of 8 weeks of induction therapy and 44 weeks of maintenance therapy with ustekinumab in adults with moderate-to-severe UC who did not respond to or were intolerant of corticosteroids, immunomodulators, 1 or more anti-TNF agents, or vedolizumab [42]. In total, 961 patients were randomly assigned to receive 1 dose of placebo, 130 mg ustekinumab, or 6 mg/kg ustekinumab intravenously. The percentage of patients with a history of any anti-TNF agent and vedolizumab failure was 17.2%, 18%, and 14.7% in the groups that received 130 mg ustekinumab, 6 mg/kg ustekinumab, and placebo, respectively. Patients who demonstrated a response to induction therapy at week 8 were randomly assigned to receive maintenance injections of placebo (175 patients) or 90 mg ustekinumab (every 12 weeks [172 patients] or 8 weeks [176 patients]) subcutaneously. The primary outcome following induction and maintenance therapy was clinical remission at weeks 8 and 44, respectively. A significantly higher percentage of patients was in clinical remission at week 8 upon treatment with 130 mg (15.6%) or 6 mg/kg (15.5%) IV ustekinumab than upon treatment with placebo (5.3%) (P<0.001 for both comparisons). Among patients who demonstrated a response to ustekinumab induction therapy and were randomized a second time, a significantly higher percentage of patients was in clinical remission at week 44 upon subcutaneous (SC) injection of 90 mg ustekinumab every 12 (38.4%) or 8 (43.8%) weeks than upon treatment with placebo (24.0%) (P=0.002 and P<0.001, respectively). The occurrence of serious adverse events, including death and malignancies, did not differ among the groups.
Data are lacking regarding dose optimization of ustekinumab in moderate-to-severe UC patients who lose their response to maintenance therapy with ustekinumab. Two analyses investigated the pharmacokinetics of ustekinumab in moderate-to-severe UC patients using data from the UNIFI trials. One investigated the association between the concentration of ustekinumab and its efficacy during induction and maintenance therapy in moderate-to-severe UC patients according to clinical effects, histologic features, and inflammation [102]. The median trough concentration was approximately 3-fold higher with dosing every 8 weeks than every 12 weeks. Serum ustekinumab concentrations were associated with both histologic and clinical features of efficacy as well as inflammation marker normalization [102]. Another study conducted population pharmacokinetics and exposure-response modeling analyses based on data from the UNIFI trials [103]. Exposure-response modeling suggested that the efficacy of 6 mg/kg IV induction ustekinumab therapy and 90 mg SC maintenance ustekinumab therapy every 8 weeks was greater than that of 130 mg IV induction ustekinumab therapy and 90 mg SC maintenance ustekinumab therapy every 12 weeks, respectively [103].
There is no recommendation for or against combination therapy with ustekinumab and immunomodulators over ustekinumab monotherapy in moderate-to-severe UC patients. Two observational studies reported contradictory results regarding whether combination therapy with an immunomodulator is superior to ustekinumab monotherapy in IBD patients [104]. One study included 363 patients (359 CD patients and 4 UC patients) who received maintenance therapy with ustekinumab and were followed up for at least 1 year. Among them, 120 patients treated with ustekinumab received combination therapy (33.1%, 57 received thiopurines and 63 received methotrexate). Patients who received combination therapy and ustekinumab monotherapy did not differ in terms of clinical response and remission at week 14 (54.6% vs. 65.8%, P=0.08), week 30 (71.6% vs. 77.4%, P=0.33), or week 54 (62.1% vs. 67.0%, P=0.52) [104]. Similar percentages of patients remained on treatment or exhibited an endoscopic response at 1 year among patients who received combination therapy or ustekinumab monotherapy [104]. However, Ma et al. [105] reported that concurrent immunomodulation was associated with a lower risk of loss of response (hazard ratio, 0.39; 95% CI, 0.17–0.92) in maintenance therapy of 104 CD patients who achieved a steroid-free response with ustekinumab induction.
The risk of tuberculosis reactivation is lower with ustekinumab than with anti-TNF agents. In the PSOLAR study including 40,388 PY, the overall IR of serious infection was higher for infliximab (2.91/100 person-years) than for ustekinumab (0.93/100 person-years) [49].
Statement 7
We recommend tofacitinib for induction of remission in patients with moderate-to-severe UC who have an inadequate response to anti-TNF agents (Strong recommendation, Moderate quality of evidence).
OCTAVE Induction 1 and 2, 2 multicenter, randomized, double-blind, placebo-controlled phase 3 trials, evaluated the efficacy of tofacitinib in terms of induction of remission in moderately-to-severely active UC patients [65]. In total, 1,139 patients were randomized (4:1) to be treated with 10 mg tofacitinib or placebo twice daily for 8 weeks. The primary endpoint and key secondary endpoint were clinical remission and mucosal healing at week 8, respectively. In OCTAVE Induction 1 and 2, the clinical remission rate at week 8 was 18.5% and 16.6% among tofacitinib-treated patients, respectively, and 8.2% and 3.6% among placebo-treated patients, respectively (P<0.01 for both comparisons) [65]. Approximately 70% of patients had a history of treatment failure with immunomodulators other than biologics or corticosteroids, 52% of patients had a history of anti-TNF agent treatment failure (56% primary non-responders), and 46% of patients were using oral corticosteroids when they entered the trials [65]. Among patients with a history of anti-TNF agent failure in OCTAVE Induction 1 and 2, 11.1% (27/243) and 11.7% (26/243) of patients who received tofacitinib were in clinical remission at week 8, respectively, compared with 1.6% (1/64) and 0% (0/60) of patients who received placebo, respectively (both comparisons were statistically significant) [65]. Moreover, for patients who had been previously exposed to anti-TNF agents, the mucosal healing rate at week 8 was significantly higher among those who received tofacitinib than among those who received placebo in both trials (OCTAVE 1: 24% vs. 6.2%, P=0.001; OCTAVE 2: 21.8% vs. 6.2%, P=0.004) [65]. These results suggest that tofacitinib effectively induces remission even in patients who have a history of anti-TNF agent failure. Meanwhile, 593 patients (45% of whom had a history of anti-TNF agent failure) who demonstrated a clinical response at the end of week 8 in OCTAVE Induction 1 or 2 following treatment with 10 mg tofacitinib twice daily were re-randomized into OCTAVE Sustain [65]. They received 5 or 10 mg tofacitinib twice daily as maintenance therapy or placebo for 52 weeks. Upon entry, 30% of patients were in remission. Clinical remission at week 52 was the primary endpoint of OCTAVE Sustain. At week 52, 34%, 41%, and 11% of patients who received 5 mg tofacitinib, 10 mg tofacitinib, and placebo were in remission, respectively. In addition, 37%, 46%, and 13% of patients who received 5 mg tofacitinib, 10 mg tofacitinib, and placebo achieved mucosal healing, respectively [65]. There was no significant difference in the efficacy of tofacitinib relative to placebo in terms of anti-TNF failure status in subgroup analysis [65].
Based on the OCTAVE program results, tofacitinib was approved for treatment of moderate-to-severe UC patients at a dose of 10 mg twice daily up to week 16 for induction of a response and 5 mg twice daily for maintenance. Tofacitinib labels state that the lowest effective dose needed to maintain a response should be used [106,107]. However, a dose of 10 mg twice daily is allowed during maintenance therapy in patients who lose their response while on 5 mg twice daily maintenance therapy, but this high-dose maintenance therapy should be used for as short a duration as possible, taking into account the benefit and risk to the individual patient. A prospective study regarding the recapture response to tofacitinib after dose escalation evaluated data from an on‐going, open‐label, long‐term extension study called OCTAVE Open [108]. Sixty-six patients who responded to tofacitinib induction therapy and were in remission following 52 weeks on 10 mg twice daily maintenance therapy were included in the dose de‐escalation group. These patients were de‐escalated to 5 mg twice daily in OCTAVE Open. Fifty-seven patients who responded to tofacitinib induction therapy and experienced treatment failure due to flare-ups while on 5 mg twice daily maintenance therapy were included in the dose escalation group. These patients were escalated to 10 mg twice daily in OCTAVE Open [108]. In the dose de‐escalation group at months 2 and 12, a clinical response was maintained in 92.4% (61/66) and 84.1% (53/63) of patients, respectively, while remission was maintained in 80.3% (53/66) and 74.6% (47/63) of patients, respectively.108 In the dose escalation group at months 2 and 12, a clinical response was recaptured in 57.9% (33/57) and 64.9% (37/57) of patients, respectively; 35.1% (20/57) and 49.1% (28/57) of patients were in clinical remission, respectively; and mucosal healing was observed in 40.4% (23/57) and 57.9% (33/57) of patients, respectively [108]. These observations suggest that, for patients who initially respond to tofacitinib but become non-responsive after dose reduction for maintenance, the response is recaptured in more than half of patients within 2 months after dose escalation to 10 mg twice daily, suggesting that a dose escalation strategy is effective and feasible. A retrospective, observational, cohort study included 134 UC patients (83% of whom had been treated with at least 1 biologic previously) who received tofacitinib in the UK. In total, 77.9% (81/104) of patients initially followed the standard induction regimen and started taking tofacitinib at a dosage of 5 mg twice daily after a median of 73 days (interquartile range, 56–99 days) [109]. Following dose reduction, symptoms recurred in 32.4% (24/74; 95% CI, 22%–44%) of patients after a median of 41 days (interquartile range, 26–91 days), and dose re-escalation recaptured the response in 47.4% (9/19; 95% CI, 25%–71%) of patients [109].
Regarding the safety of tofacitinib in UC patients, a recent study analyzed pooled data from UC patients in phase 2 and 3 trials and an open-label extension who received tofacitinib. It showed that the safety profile was similar to that reported for rheumatoid arthritis patients and UC patients who received other biologics, except for a dose-dependent higher IR of herpes zoster infections [110].
Statement 8
We recommend that currently approved anti-TNF biosimilars can be used for induction and maintenance of remission in patients with moderate-to-severe UC (Strong recommendation, Low quality of evidence).
Biosimilars are complex protein-based biological products that are highly similar, but are not identical, to an approved originator and whose safety, purity, and potency do not differ in a clinically meaningful way [111]. Manufacture of biosimilars leads to many opportunities for minor variations in their structures to arise because biologic products often have large, complex, protein-based molecular structures [111]. Differences in the manufacturing process can also change the biologic properties of the molecule, and even affect the function and immunogenicity of a biosimilar. Therefore, biosimilars must be approved through analytical, animal, clinical pharmacology, and clinical studies [111]. The first biosimilar of infliximab, called CT-P13, has a similar pharmacokinetic profile as originator infliximab. Two phase 3 trials showed that the efficacy, toxicity, and immunogenicity of CT-P13 and originator infliximab are similar in remission and maintenance therapy for rheumatoid arthritis (PLANETRA trial) and ankylosing spondyloarthritis (PLANETAS trial) patients, respectively [112-115]. Similar phase 3 trials were conducted of biosimilars of infliximab, namely, SB2 [116-118], PF-06438179/GP1111 [119], and ABP 710 [120], in rheumatoid arthritis. The efficacy, safety, and immunogenicity profiles of these biosimilars were comparable with those of the originator. The same approach was used to assess ZRC-3197 [121], ABP 501 [122,123], BI 695501 [124], GP2017 [125,126], SB5 [127,128], PF-06410293 [129], and FKB327 [130], which are biosimilars of adalimumab, in rheumatologic and dermatologic diseases.
In a prospective cohort study from Hungary, 84 consecutive UC patients who started taking an infliximab biosimilar, CTP13, had a clinical response rate of 77.6% and a clinical remission rate of 58.6% at week 14 with comparable infusion reactions (6.6%) and infectious adverse events (5.7%) [131]. Patients who were infliximab-naïve had a significantly higher clinical remission rate at week 14 than patients who had been exposed to infliximab (65.1% vs. 33.3%, P<0.05) [131]. In another prospective cohort study from Italy (PROSIT-BIO cohort), a subset of consecutive IBD patients had not been exposed to anti-TNF agents (n = 311) or had been previously exposed to biologics (n = 139). At weeks 8 and 24, CT-P13 yielded a clinical remission rate of 95.7% and 73.7% in naïve patients, respectively, and 97.2% and 62.2% in pre-exposed patients, respectively [132]. The expanded PROSIT-BIO cohort showed that the safety and efficacy of CT-P13 were consistent with those of originator infliximab in IBD patients at 12 months, although CT-P13 and originator infliximab were not directly compared [133]. In a recent meta-analysis up to January 2019 that included 1 RCT and 15 observational studies of CT-P13-treated UC patients, the pooled clinical response rate was 0.68 (95% CI, 0.63–0.72) and 0.54 (95% CI, 0.45–0.63) at weeks 8–14 and 48–63, respectively, and safety was tolerable [134]. Although CT-P13 and originator infliximab have not been directly compared in UC patients, the PALETCD study of CD patients showed that the clinical response, clinical remission, and adverse events were comparable in the 2 groups up to week 30 [135].
A SC CT-P13 formulation (CT-P13 SC) was recently developed that contains 120 mg/mL infliximab. A phase I study of CT-P13 SC was conducted with active IBD patients who were naïve to anti-TNF agents [136]. Sixty-six patients (28 CD patients and 38 UC patients) and 65 patients (25 CD patients and 40 UC patients) were randomly assigned to CT-P13 SC group and CT-P13 IV group at week 6, respectively, after they had been treated with CT-P13 IV at weeks 0 and 2. In terms of drug concentration at week 22, CT-P13 SC was not inferior to CT-P13 IV, and the efficacy, safety, and immunogenicity outcomes did not differ between the 2 groups up to week 30. Patients receiving CT-P13 IV were switched to CT-P13 SC at week 30. Their efficacies and safety profiles were comparable in patients who received CT-P13 SC throughout and patients who switched from CT-P13 IV up to week 54 [136]. Two phase 3 trials are currently ongoing and recruiting patients with UC (NCT04205643) and CD (NCT03945019).
With regard to adalimumab biosimilars, as with infliximab, there are no direct comparative studies in UC patients. However, efficacy, safety, and immunogenicity in 147 moderate-to-severe CD patients were compared in a recent phase 3 RCT (VOLTAIRE-CD). It showed that the efficacy and safety of BI 695501 were similar to those of the reference product up to week 24 [137]. Small retrospective studies in India showed that ZRC-3197 is a cost-effective and safe alternative to originator adalimumab for moderate-to-severe UC patients [138,139].
Statement 9
We recommend that patients with UC receiving originator anti-TNF agents can be switched to currently approved antiTNF biosimilars if they are stable (Strong recommendation, Moderate quality of evidence).
The NOR-SWITCH non-inferiority trial enrolled consecutive patients with immune-mediated diseases such as CD (n = 155) and UC (n = 93) who were receiving originator infliximab to maintain remission (at least 6 months). They were randomized to continue receiving originator infliximab or switch to CT-P13 up to week 52 [140]. Among patients who continued to receive originator infliximab or switched to CT-P13, disease worsened in 21.2% and 36.5% (95% CI, –29.3 to 0.7) of CD patients, respectively, and in 9.1% and 11.9% (95% CI, –15.2 to 10.0) of UC patients, respectively [140]. The frequency of adverse events in originator infliximab-treated patients was comparable with that in CT-P13-treated patients. The NOR-SWITCH extension trial assessed efficacy, safety, and immunogenicity in 197 patients (including 65 CD patients and 42 UC patients) on CT-P13 maintenance therapy and 173 patients (including 62 CD patients and 38 UC patients) who switched from originator infliximab to CT-P13 at week 52 during the 78 week study period [141]. Disease worsened in 20.6% and 13.1% of patients in the maintenance and switch groups for CD (95% CI, –5.2 to 21.0), respectively, and in 15.4% and 2.9% of patients in the maintenance and switch groups for UC (95% CI, –0.1 to 25.0), respectively, while the safety profile was comparable between the arms [141]. A subset of CD patients in the NOR-SWITCH main trial exhibited a difference that favored the originator and was close to being significant. However, subgroup analysis of CD and UC patients in the NOR-SWITCH main and extension trials showed that the efficacy, safety, and immunogenicity of the originator were comparable with those of CT-P13 in CD and UC patients [142].
In the PROSIT-BIO study, safety and efficacy did not differ between 97 IBD patients who switched to CT-P13 after receiving a mean of 18 originator infliximab infusions and 311 anti-TNF agent-naïve IBD patients who received CT-P13 [132]. In the expanded PROSIT-BIO cohort, safety and efficacy did not differ between 155 patients who switched to CT-P13 after receiving a mean of 17 originator infliximab infusions and naïve patients at 12 months [133]. In a prospective study of a Swedish cohort of 313 IBD patients (195 CD patients and 118 UC patients) who switched to CT-P13 from the originator, clinical disease activity index, quality of life, biomarkers, trough levels of drugs, and the proportion of patients in remission did not significantly change compared with the baseline (time of switch) and the risk of adverse events did not increase during 12 months of follow-up [143].
With regard to currently approved adalimumab biosimilars, data regarding switching to a biosimilar from the originator are lacking in UC patients. The VOLTAIRE-CD trial recently showed that efficacy and safety in moderate-to-severe CD patients who switched to BI 695501 from the reference product were comparable to those in patients in whom the reference product was used for maintenance therapy [137]. A recent small, prospective, multicenter study of IBD patients reported that safety and efficacy were comparable in patients who switched to ABP501 or SB5 from the originator and patients who did not switch [144].
Statement 10
We recommend infliximab or cyclosporine as a rescue therapy in patients with ASUC who are refractory to IV corticosteroids (Strong recommendation, Moderate quality of evidence).
A small Swedish-Danish RCT compared placebo and infliximab as rescue therapy in 45 patients with ASUC that was refractory to 4 days of treatment with IV corticosteroids. A single dose of 5 mg/kg infliximab (rate of surgery, 29% [7/24]) was superior to placebo (rate of surgery, 67% [14/21]) for reducing the colectomy risk within 90 days of hospitalization (RR, 0.44; 95% CI, 0.22–0.87) [145]. Long-term follow-up showed the sustained benefit of infliximab (rate of surgery, 50% [12/24]) over placebo (rate of surgery, 76% [16/21]) at 3 years [146].
A French parallel, open-label RCT called the CYSIF trial compared the efficacy of cyclosporine (n = 58; 2 mg/kg/day for 1 week, followed by oral drug) and infliximab (n = 57; 5 mg/kg on days 0, 14, and 42) in patients with ASUC that was refractory to high-dose IV corticosteroids [147]. Occurrence of treatment failure, including the absence of clinical response or steroidfree remission, relapse, and severe adverse events, within 3 months did not differ between the 2 groups (60% vs. 54%, respectively; 95% CI, –7 to 19; P=0.52) [147]. Additionally, colectomy-free survival was similar in the 2 groups (17% vs. 21%, respectively, P=0.60) [147]. In a retrospective study from Korea, the colectomy rate in patients with corticosteroid-refractory UC who received infliximab (n = 33) and cyclosporine (n = 10) was 3% and 30% up to 12 months, respectively [148]. However, the Cox proportional hazard model showed that rescue therapy was not an independent associated factor and did not find a difference between the groups (hazard ratio, 0.166; 95% CI, 0.013–2.088; P=0.164) in terms of prevention of colectomy [148]. Another recent Korean single-center retrospective study also showed that the cyclosporine group (n = 23) and the infliximab group (n = 98) did not show significant differences in the cumulative rates of treatment failure (39.1% vs. 34.7%, P=0.714) and colectomy (26.1% vs. 13.3%, P=0.198) at 3 months in patients with corticosteroid-refractory ASUC [149]. In a mixed methods, open-label, pragmatic randomized trial from the UK, 270 ASUC patients received cyclosporine (n = 135) or infliximab (n = 135) [150]. Quality-adjusted survival (P=0.603), which was the primary outcome, and Crohn’s and Ulcerative Colitis Questionnaire scores, EQ-5D, and SF-6D scores, which were the secondary outcomes, were similar in the 2 groups. Furthermore, the colectomy rate (48% for cyclosporine-treated patients vs. 41% for infliximab-treated patients, P=0.223) and mean time to colectomy (744 days for cyclosporine-treated patients vs. 811 days for infliximab-treated patients) were similar in the 2 groups.
There is long-term persistence on infliximab therapy. By contrast, most patients only receive cyclosporine for a limited amount of time and subsequently switch to immunomodulators or anti-TNF agents as a long-term maintenance therapy. In a recent case series, vedolizumab was used as a maintenance therapy in corticosteroid-refractory UC patients who were in remission with co-induction with cyclosporine and vedolizumab for 6–12 weeks and had been exposed to infliximab [151]. In total, 73% (8/11) and 45% (5/11) of patients achieved a clinical response by week 14 and clinical remission by week 52, respectively. However, data regarding the efficacy of maintenance therapy with vedolizumab in this patient population are very limited.
Retrospective observational studies that investigated the benefit of accelerated over standard infliximab dosing in ASUC patients reported different results. In 1 study, 132 patients received standard infliximab therapy (5 mg/kg infliximab at weeks 0, 2, and 6) and 81 patients received accelerated infliximab therapy (> 5 mg/kg infliximab at shorter intervals) [152]. Rates of in-hospital colectomy (8% vs. 9%; adjusted OR, 1.35; 95% CI, 0.38–4.82) and colectomy during 24 months of follow-up (P> 0.20) did not differ between the groups [152]. Another study of ASUC compared 58 patients receiving accelerated dosing of infliximab and 87 patients receiving standard dosing (32 in the historical group [2010–2013] and 55 in the current group [2014–2017]) [153]. Time to colectomy was shorter in the historical standard dosing group (log-rank P=0.0013), but was similar in the accelerated dosing and current standard dosing groups (log-rank P=0.32) [153]. The authors concluded that time to colectomy is significantly prolonged with accelerated dosing of infliximab in selected patients with more severe disease. In propensity score based analyses, 1 study of 52 ASUC patients reported that the 30 day colectomy rate (57% vs. 27%, P=0.048) and index admission colectomy rate (53% vs. 23%, P=0.045) were higher among patients on standard induction than among those on accelerated induction, but the overall colectomy rates at 12 months did not differ between the 2 groups (57% vs. 31%, P=0.09) [154]. However, another study of 42 ASUC patients reported that patients who received accelerated induction with infliximab required colectomy more rapidly within 30 days than those in the non-accelerated group (P=0.001) [155].
A few recent case series reported the efficacy of rescue therapy with tofacitinib for ASUC patients in whom IV corticosteroids fail [156-158]. Post-hoc analysis of OCTAVE 1 and 2 suggested that stool frequency and rectal bleeding may decrease in about one-third of moderate-to-severe UC patients within 3 days of therapy commencement [159]. In the GETAID cohort in France, salvage therapy with tofacitinib (10 mg twice daily) was evaluated in 55 hospitalized patients who had refractory ASUC (median follow-up of 6.5 months) [160]. The incidence of colectomy at month 3 was estimated to be 21.1% in the GETAID cohort, which is comparable with that in patients treated with infliximab or cyclosporine in the CYSIF trial. However, data concerning the efficacy of tofacitinib in ASUC patients are very limited and the level of evidence is very low. Prospective comparative studies investigating the effect and safety of tofacitinib in ASUC patients are needed.
Statement 11
We suggest that discontinuation of anti-TNF agents is not mandatory prior to surgery in patients with moderate-to-severe UC (Conditional recommendation, Low quality of evidence).
There is a controversy about the direct associations between biologics and postoperative complications. Several retrospective studies have been conducted. Lau et al. [161] reported that increasing preoperative serum levels of anti-TNF agents were associated with unfavorable postoperative outcomes in CD patients, but not in UC patients. Zittan et al. [162] also reported that preoperative anti-TNF agent therapy was not associated with an elevated risk of infectious and noninfectious complications, including pelvic abscesses, leaks, and wound infections, in UC patients following ileal-pouch anal anastomosis. Ward et al. [163] studied Hospital Episode Statistics data from the National Health Service of England. They did not find any association between preoperative anti-TNF agent therapy and postoperative complications in UC patients who underwent subtotal colectomy. Novello et al. [164] reported that exposure to biologics including infliximab, adalimumab, and vedolizumab was not associated with increased morbidity in a matched case-control study. Abelson et al. [165] performed a study using the New York State Department of Health Statewide Planning and Research Cooperative System database. They suggested that since the introduction of biologics, patients undergoing surgery had a worse postoperative morbidity during the index hospitalization and after 90 days and 1 year of follow-up. However, this study compared postoperative outcomes not according to the use of biologics, but only the operation years.
Yang et al. [166] conducted a meta-analysis of case series in 2009, soon after the introduction of infliximab, and found that preoperative treatment with infliximab increased patients’ risk of short-term postoperative complications. However, based on a meta-analysis in 2013 including 14 observational studies (CD in 6 studies and UC/IBD-unspecified in 8 studies), Billioud et al. [167] reported that preoperative anti-TNF agent treatment marginally increased overall postoperative complications in IBD patients, especially infectious complications in CD patients, but not in UC patients. In total, 41% and 33% of patients who received and did not receive anti-TNF agent therapy developed postoperative complications, respectively, and this difference was not significant (OR, 1.32; 95% CI, 0.94–1.84) [167]. Furthermore, the percentages of patients with infectious (21% vs. 16%; OR, 1.31; 95% CI, 0.55–3.07) and noninfectious (33% vs. 24%; OR, 1.60; 95% CI, 0.85–3.00) complications were similar in the 2 groups [167]. A prospective, multicenter, pilot study by El-Hussuna et al. [168] suggested that anti-TNF agents do not affect the surgical stress response or increase postoperative complications.
Although there is controversy about direct associations between biologics and postoperative complications and the level of evidence was low from the reviewed studies, preoperative anti-TNF use in UC patients may not be significantly associated with an increase of postoperative complications.
CONCLUSIONS
Anti-TNF agents, vedolizumab, ustekinumab, and tofacitinib showed efficacy and safety for induction and maintenance of remission in patients with moderate-to-severe UC. Although prospective comparative trials are needed in the future for consensus, infliximab showed efficacy in ASUC, and perioperative use of anti-TNF agents may not increase the risk of complications. Anti-TNF biosimilars also showed comparable efficacy and safety to originator anti-TNF agents. UC is still incurable, however, many clinical studies are in progress, and novel biologics and small molecules will provide more treatment opportunities and improve quality of life for moderate-to-severe UC patients. They also will offer more therapeutic options for physicians. These third guidelines have been developed based on recently published high quality clinical data with reference to the major relevant guidelines of other countries and with consideration of the situation in Korea. They may help physicians choose evidence-based treatment options to manage moderate-to-severe UC patients.
Notes
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
Keum B is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Data Availability Statement
Not applicable.
Author Contribution
Conceptualization: Na SY, Choi CH, Kim JS. Data curation: Na SY, Choi CH, Song EM, Bang KB, Park SH, Kim ES, Park JJ, Keum B, Lee CK, Lee BI, Ryoo SB. Formal analysis: Choi M. Investigation: Choi CH. Methodology: Choi M. Project administration: Na SY, Choi CH, Kim JS. Supervision: Choi CH, Kim JS. Validation: Choi CH, Bang KB, Park SH, Kim ES, Park JJ, Keum B, Lee CK, Lee BI, Ryoo SB. Writing - original draft: Na SY, Song EM, Bang KB, Park SH, Kim ES, Park JJ, Keum B, Lee CK, Lee BI, Ryoo SB, Koh SJ, Choi M. Writing - review & editing: all authors. Approval of final manuscript: all authors.
Non-Author Contribution
The draft was reviewed and approved by the external advisory committee: Kang-Moon Lee (St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea), Won Moon (Kosin University College of Medicine), Geom Seog Seo (Wonkwang University School of Medicine), Jong Pil Im (Seoul National University College of Medicine), Sang-Bum Kang (Daejeon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea).
Supplementary Material
Supplementary materials are available at the Intestinal Research website (https://www.irjournal.org).
Supplementary Table 1. Evidence Table of Primary Randomized Controlled Trials
ir-2022-00007-Supplementary-Table-1.pdfSupplementary Table 2. Risk of Bias of Primary Randomized Controlled Trials
ir-2022-00007-Supplementary-Table-2.pdfSupplementary Table 3. Evidence Table of Primary Observational Studies
ir-2022-00007-Supplementary-Table-3.pdfSupplementary Table 4. Risk of Bias of Primary Observational Studies
ir-2022-00007-Supplementary-Table-4.pdfREFERENCES
ir-2022-00007-Supplementary-1.pdf