|
 |
- Search
| Intest Res > Volume 13(3); 2015 > Article |
|
Abstract
Background/Aims
Early use of biologics in patients with Crohn's disease (CD) improves quality of life. However, the effects of the early use of immunomodulators on long-term outcomes remain unclear. This study aimed to evaluate the effects of immunomodulators in patients with CD.
Methods
Between January 2004 and December 2011, 47 biologic-naive CD patients treated with thiopurines alone for remission maintenance were analyzed. The patients were classified into 2 groups depending on the presence or absence of digestive complications. We evaluated the efficacy of and predictive factors for thiopurine use for remission maintenance.
Results
The cumulative relapse rates at 24 and 60 months were 13.7% and 35.4%, respectively. Regarding patient characteristics, there was a significant difference in patient history of surgery between the non-relapse and relapse groups (P=0.021). The cumulative relapse rate was lower in patients without a history of surgery than in those with such a history (27.2% and 52.9% at 60.0 months, respectively). Multivariate analysis suggested that the prevalence of stricturing and penetrating complications is an independent factor for relapse. The cumulative relapse rate in patients without a history of surgery was significantly lower in the non-stricturing and non-penetrating group than in the stricturing and penetrating group (11.8% at 85.0 months vs. 58.5% at 69.0 months; P=0.036).
Crohn's disease (CD) is a chronic relapsing gastrointestinal inflammatory disorder that is characterized by frequent complications, such as stenosis and fistula, and an impaired quality of life.1 Therefore, long-term treatments are necessary for the maintenance of clinical remission in patients with CD. Conventional therapies for active CD include corticosteroid treatments.2,3,4,5,6 Recently, several randomized controlled trials demonstrated that treatment with tumor necrosis factor (TNF)-α antagonists improved the management of refractory CD.5,6 Considering the efficacy of TNF-α antagonists, in particular, a study suggested that the early intensive therapy with TNF-α antagonists might reduce the complications associated with conventional CD treatment and thus improve the quality of life.7 Therefore, early intensive therapy with these biologic agents has been proposed and is referred to as the "top-down" therapy. Although several studies emphasize the importance of early medical treatment in patients with CD, the associations between TNF-α antagonist therapy and the development of serious life-threatening infections in the early years of therapy as well as other well-documented hematologic, immunologic, cardiovascular, and malignant adverse effects are serious concerns.8,9
Thiopurines, such as azathioprine (AZA) and 6-mercaptopurine (6MP), are widely used for managing steroid-refractory IBD. However, it remains unclear whether the early use of these drugs affects long-term clinical remission and decreases the surgical rates in patients with CD.10
In the present study, we evaluated the efficacy and safety of thiopurine treatment for long-term clinical remission in Japanese biologic-naive CD patients.
We retrospectively reviewed the medical records of 531 patients with IBD who were treated and followed up between January 2004 and December 2012 at Kyoto University Hospital. The 531 patients with IBD comprised 175 CD patients, 321 UC patients, 11 Behçet's disease patients, and 24 IBD-unclassified patients. Of the 175 CD patients, 148 who received medical treatment for induction of clinical remission were enrolled in this study. The course of CD was determined before and during treatment with thiopurine. Patients with CD who met all the following criteria were enrolled in this study: (1) thiopurine treatment for over 6 months, (2) remission for at least 6 months without corticosteroid use or granulocyte-monocyte adsorption apheresis (GMAA), (3) no surgical treatment for remission, and (4) no tacrolimus or anti-TNF-α treatment. Patients who had received or were receiving 5-aminosalicylic acid drugs were not excluded. Depending on the prevalence of digestive complications, patients with CD were classified into the following 2 groups according to Montreal classification: B1 (non-stricturing and non-penetrating) and B2+B3 (stricturing and penetrating) groups.11 This retrospective, observational, single-center study was conducted in accordance with the principles of the Declaration of Helsinki and was reviewed and approved by the institutional review boards of Kyoto University Hospital.
Biologic-naive patients with CD were treated with thiopurines alone for the maintenance of clinical remission. The thiopurine dose was adjusted according to the number of white blood cells (3,000-5,000/µL) and the concentration of 6-thioguanine (6TGN) (235-400 pmol/8×108 red blood cells).12
Clinical remission was defined as a CD activity index (CDAI) score of <150 points under corticosteroid- and GMAA-free conditions.13 Relapse of CD was defined as a CDAI score of >150 points or an increase in the CDAI score by 100 points from baseline.13,14 Endoscopic activity of CD was assessed using the simple endoscopic score for CD.15 Mucosal healing was defined as the score of 0. Intolerance to thiopurine was defined as a reaction occurring within a month after the induction of thiopurine, including pancreatitis, abdominal pain, fever, arthralgia, myalgia, cutaneous rash, fatigue, alopecia, hepatitis, and digestive intolerance.16 Data on the following variables were collected: age at CD diagnosis, gender, disease location, disease behavior, duration before thiopurine initiation, smoking, CDAI, and CRP level at the time of induction of thiopurine treatment.
The primary outcome was the cumulative relapse rate in patients with CD. The secondary outcomes included the identification of factors associated with the risk of relapse and safety of long-term treatment with AZA/6MP in patients with CD. The safety of thiopurine treatment was assessed by evaluating the adverse event and mortality rates associated with the treatment, including malignancies. Categorical and continuous data were compared using a two-tailed Fisher exact test and Mann-Whitney U test. The cumulative relapse rates were evaluated using Kaplan-Meier analysis (log-rank test). Multivariate analysis of the time to relapse in patients with CD was performed using Cox regression analysis. The following variables were analysed as potential predictors of relapse in CD patients: age, CRP level, stricturing (B2) and penetrating (B3), and the time to initiate thiopurine treatment after CD diagnosis. A value of P<0.05 was considered statistically significant.
One hundred forty-eight patients with CD (mean age, 30 years [range, 14-71 years]; men: 114, women: 34) were retrospectively enrolled in this study. Of the 148 patients with CD, 81 were biologic-naive. Of the 81 biologic-naive patients, 47 (58.0%) who were treated with thiopurines alone for the maintenance of clinical remission with corticosteroids or GMAA were analyzed. Almost all of these 47 patients were male (87.2%; Table 1). The median age at diagnosis of CD was 26 years, and almost all the patients were <40 years of age. The median disease duration was 6 months (1-264 months). Of the 47 patients, 33 (70.2%) had ileal lesions (L1+L3). Based on the Montreal classification of CD, disease behavior in patients was classified as follows: non-stricturing and non-penetrating (B1): 22 patients (46.8%); stricturing (B2): 17 patients (36.2%); penetrating (B3): 1 patient (2.1%); and perianal disease modifier (B4): 10 patients (21.3%). 12 patients (25.5%) were smokers. The median CDAI score at induction of thiopurine was 200, and the median CRP level was 0.8 mg/dL. Moreover, 14 patients (29.8%) had a history of surgery. Of the 47 patients, 41 were treated with AZA, and the remaining 6 were treated with 6MP. Moreover, 3 of the 47 patients were treated with concomitant allopurinol for optimizing the thiopurine dose. The median AZA dose was 50 mg (25-100 mg), and the median 6MP dose was 20 mg (10-50 mg). Moreover, the median cumulative dose of AZA in this observational period was 54,000 mg and that of 6MP was 20,835 mg. Moreover, in 14 of 47 patients, the 6TGN level was evaluated, and the median 6TGN level was 405 pmol/8×108 red blood cells. The median duration of thiopurine use during the follow-up period was 39.5 months (13-85 months).
Of the 47 patients, 13 experienced a relapse. Ten of those 13 patients experienced an exacerbation of symptoms related with CD. The remaining 3 patients experienced ileus due to intestinal stricture followed by surgery. The time-to-relapse curves are shown in Fig. 1. The median follow-up time was 40.75 months. Based on the Kaplan-Meier analysis, the overall cumulative relapse rates of the 47 patients with CD at 12, 24, and 60 months were 0%, 13.7%, and 35.4%, respectively (Fig. 1).
To analyze the risk factors of relapse in biologic-naive CD patients receiving long-term thiopurine treatment, we evaluated the differences in patient characteristics between the non-relapse and relapse groups (Table 2). Among the patient characteristics, patient history of surgery significantly differed between the non-relapse and relapse groups (P=0.021). Next, we analyzed the cumulative relapse rates in biologic-naive CD patients with and those without a history of surgery. As shown in Fig. 2, the cumulative relapse rates of biologic-naive CD patients with and those without a history of surgery were 52.9% at 77.6 months and 27.2% at 85.0 months, respectively (P=0.071). These data suggest that a history of surgery could affect the relapse rates of biologic-naive CD patients treated with thiopurine, although no significant difference was observed in the cumulative relapse rates of biologic-naive CD patients with and those without a history of surgery.
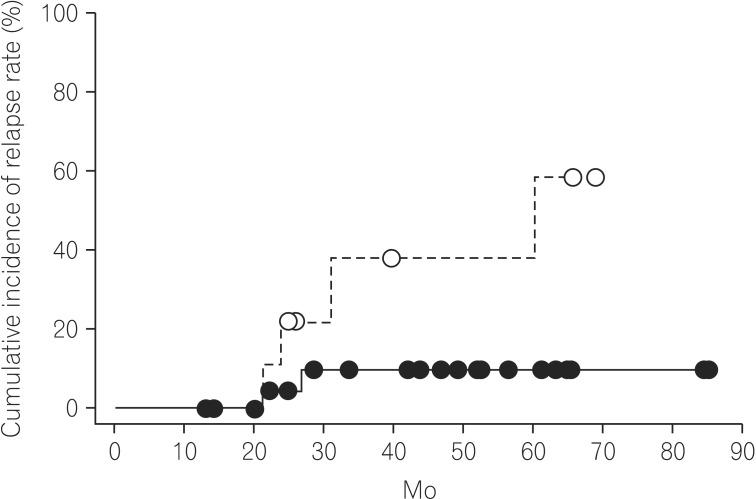
To exclude the influence of patient history of surgery on thiopurine treatment, we analyzed the efficacy of thiopurines in biologic-naive CD patients without a history of surgery. To evaluate the predictive factors for CD relapse in biologic-naive CD patients without a history of surgery, we assessed the differences in the patient characteristics between the non-relapse and relapse groups. As shown in Table 3, the non-relapse group had a higher number of patients with the B1 phenotype than the relapse group. In accordance with Fig. 2, we consider that initiating thiopurine treatment before the development of digestive complications requiring surgery might be important for the long-term maintenance of remission in biologic-naive CD patients. Therefore, according to the prevalence of digestive complications, CD patients without a history of surgery were classified as follows: B1 and B2+B3 group. Based on the Kaplan-Meier analysis, the cumulative relapse rate in biologic-naive CD patients without a history of surgery was significantly lower in the B1 group than in the B2+B3 group (11.1% at 85.0 months vs. 58.5% at 69.0 months, P=0.036; Fig. 3).
To evaluate the clinical factors associated with the long-term maintenance of remission with thiopurine treatment, we performed multivariate analysis of the time to relapse in biologic-naive CD patients without a history of surgery. However, Cox regression analysis could not identify the risk factors of relapse in the biologic-naive CD patients without a history of surgery after remission maintenance with thiopurines (Table 4). Therefore, in accordance with Table 2 and Fig. 2, we consider that thiopurine use would be beneficial for the long-term maintenance of remission in biologic-naive CD patients without digestive complications and a history of surgery.
Adverse events occurred in 7 of the 47 patients treated with thiopurines (14.9%; data not shown). Leukopenia, liver dysfunction, and nausea occurred in 2 patients each, and pancreatitis occurred in the remaining patient. All patients recovered after decreasing the thiopurine dose or stopping thiopurine treatment. Moreover, none of the patients treated with thiopurines reported malignancies or life-threatening infections.
Our current study shows that thiopurine treatment could lead to a clinically favorable outcome in biologic-naive CD patients, and thiopurine use before the development of digestive complications led to reduced rates of relapse and surgery. To our knowledge, this is the first study to demonstrate that thiopurine use may contribute to the long-term maintenance of remission in Japanese biologic-naive CD patients.
The superiority of thiopurine immunomodulators in inducing, and particularly maintaining, remission of CD has been documented.17,18 However, there are limited data on the long-term effects of thiopurines in CD patients. First, we evaluated the effects of thiopurines on clinical remission maintenance in biologic-naive patients, which demonstrated that the cumulative relapse rates at 1, 3, and 5 years were 0%, 13.7%, and 35.4%, respectively. Several studies have reported the efficacy of thiopurine treatment in improving the long-term clinical outcomes in CD patients; therefore thiopurine treatment is likely to be effective in biologic-naive CD patients.19,20,21,22 However, the SONIC (The study of Biologic and Immunomodulator Naive Patients in CD) study demonstrated a greater clinical benefit from infliximab than from AZA alone in biologic-naive CD patients, because CD patients who received infliximab monotherapy and infliximab plus AZA combination therapy had higher rates of clinical remission and mucosal healing than those who received AZA monotherapy.5 Moreover, Kwak et al. recently reported the efficacy of the early induction of immunomodulators in biologic-naive CD patients.23 They showed that in biologic-naive CD patients who received thiopurine treatment within 6 months after diagnosis, the cumulative relapse rates at 1, 2, and 3 years were 7%, 22.3%, and 60.9%, respectively. Moreover, there were no significant differences in the relapse rates between the early induction and conventional therapy groups. Therefore, further studies are necessary to confirm the efficacy of thiopurine induction before starting biologic treatment for improving long-term clinical outcomes in biologic-naive CD patients.
Recently, 2 studies demonstrated that very early induction of AZA treatment was not effective in the corticosteroid-free clinical remission of patients with newly diagnosed CD.10,24 However, these data conflict with the results of other studies. In fact, the GETAID (Groupe d'Etude Therap-utique des Affections Inflammatoires du tube Digestif) reported that approximately 60% of the CD patients in the conventional management group required immunosuppressive drugs for the corticosteroid-free clinical remission in the 3-year study period.24 Moreover, the occurrence of perianal lesions was significantly lower in the AZA-treated group than in the conventional treatment group.24 In addition, the AZTEC (AZA for Treatment of Early CD in Adults) study showed that early AZA use was more effective in preventing moderate-to-severe relapses than placebo use in the post hoc analysis.10 Moreover, D'Haens et al. demonstrated that combined immunosuppression was effective for induction of remission and reduction of corticosteroid use in early diagnosed CD patients.25 Therefore, these studies do not necessarily deny the effectiveness of thiopurine treatment in CD patients but suggest the importance of identifying when thiopurine treatment must be started in CD patients in whom disease activity becomes progressive without optimal immunosuppressive treatments.
The most recent Cochrane Library meta-analysis reported that thiopurines are effective for the maintenance of remission, and another meta-analysis reported the efficacy of thiopurines in the prevention of postoperative recurrences.17,26 Moreover, a population-based inception cohort from Hungary showed a positive correlation between early thiopurine treatment and a reduction in surgical rates.19,27 Our data revealed that the cumulative relapse rates were lower in biologic-naive CD patients without a history of surgery than in those with such a history, although this result was not significant (P=0.071). Considering the natural course of CD,1 a history of surgery is a reasonable risk factor for CD relapse in patients treated with thiopurines.28,29 However, in CD patients without a history of surgery, our multivariate analysis revealed that the prevalence of digestive complications is a possible independent risk factor for CD relapse. In biologic-naive CD patients without a history of surgery, the cumulative relapse rate was lower in patients without digestive complications than in those with them. Taken together, the early induction of thiopurines before the development of digestive complications could contribute to better clinical outcomes in CD patients. However, our study had several limitations including a small sample size, heterogeneity in patient characteristics, and a lack of comparison groups. Therefore, further studies with larger sample sizes are required to confirm our data.
Finally, we evaluated the adverse events associated with long-term thiopurine treatment. The frequency of adverse events in the present study was similar to that reported by previous Western studies.30,31 Moreover, all patients recovered after the thiopurine dose was decreased. The American Gastroenterological Association has promoted the usefulness of thiopurine metabolite monitoring for optimizing thiopurine treatment in IBD patients to avoid adverse events.31 The frequency and phenotypes of mutations of thiopurine methyltransferase, which is associated with thiopurine metabolism, differ between Japanese and Western patients.32 Therefore, adequate monitoring of the concentration of the AZA metabolite 6TGN is necessary and helpful for the long-term maintenance of remission and for maximizing the efficacy and minimizing the toxicity of the thiopurine drug in Japanese CD patients.
In conclusion, our data demonstrated that thiopurine treatment might be useful for the long-term maintenance of remission in biologic-naive CD patients without digestive complications and a history of surgery. Because the use of biologics has just been in its golden age in the field of IBD treatments, we must recognize the role of thiopurine agents in patients with biologic-naive CD.
NOTES
Financial support: This work was supported by Grants-in-aid for Scientific Research (25130706, 24229005, 24659363, and 24590941) from the Japan Society for the Promotion of Science (JSPS) and Health and Labor Sciences Research Grants for Research on Rare and Intractable Diseases from the Ministry of Health, Labor, and Welfare, Japan.
References
1. Pariente B, Cosnes J, Danese S, et al. Development of the Crohn's disease digestive damage score, the Lémann score. Inflamm Bowel Dis 2011;17:1415-1422.PMID: 21560202.


2. Anthonisen P, Barany F, Folkenborg O, et al. The clinical effect of salazosulphapyridine (Salazopyrin r) in Crohn's disease. A controlled double-blind study. Scand J Gastroenterol 1974;9:549-554.PMID: 4153646.


3. Travis SP, Stange EF, Lémann M, et al. European evidence based consensus on the diagnosis and management of Crohn's disease: current management. Gut 2006;55(Suppl 1): i16-i35.PMID: 16481629.



4. Lichtenstein GR, Hanauer SB, Sandborn WJ. Practice Parameters Committee of American College of Gastroenterology. Management of Crohn's disease in adults. Am J Gastroenterol 2009;104:465-483.PMID: 19174807.


5. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med 2010;362:1383-1395.PMID: 20393175.


6. Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology 2007;132:52-65.PMID: 17241859.


7. Peyrin-Biroulet L, Oussalah A, Williet N, Pillot C, Bresler L, Bigard MA. Impact of azathioprine and tumour necrosis factor antagonists on the need for surgery in newly diagnosed Crohn's disease. Gut 2011;60:930-936.PMID: 21228429.


8. Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor a-neutralizing agent. N Engl J Med 2001;345:1098-1104.PMID: 11596589.


9. Mackey AC, Green L, Liang LC, Dinndorf P, Avigan M. Hepatosplenic T cell lymphoma associated with infliximab use in young patients treated for inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2007;44:265-267.PMID: 17255842.


10. Panés J, López-Sanromán A, Bermejo F, et al. Early azathioprine therapy is no more effective than placebo for newly diagnosed Crohn's disease. Gastroenterology 2013;145:766-774.PMID: 23770132.


11. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006;55:749-753.PMID: 16698746.


12. Sandborn WJ. Rational dosing of azathioprine and 6-mercaptopurine. Gut 2001;48:591-592.PMID: 11302950.



13. Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology 1976;70:439-444.PMID: 1248701.


14. Van Assche G, Dignass A, Reinisch W, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: Special situations. J Crohns Colitis 2010;4:63-101.PMID: 21122490.



15. Daperno M, D'Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc 2004;60:505-512.PMID: 15472670.


16. Stange EF, Travis SP, Vermeire S, et al. European evidence-based Consensus on the diagnosis and management of ulcerative colitis: Definitions and diagnosis. J Crohns Colitis 2008;2:1-23.PMID: 21172194.



17. Prefontaine E, Sutherland LR, Macdonald JK, Cepoiu M. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev 10.1002/14651858.CD000067.pub2. Published online 21 January
2009.

18. Markowitz J, Grancher K, Kohn N, Lesser M, Daum F. A multicenter trial of 6-mercaptopurine and prednisone in children with newly diagnosed Crohn's disease. Gastroenterology 2000;119:895-902.PMID: 11040176.


19. Lakatos PL, Golovics PA, Dávid G, et al. Has there been a change in the natural history of Crohn's disease? Surgical rates and medical management in a population-based inception cohort from Western Hungary between 1977-2009. Am J Gastroenterol 2012;107:579-588.PMID: 22233693.



20. Bouhnik Y, Lémann M, Mary JY, et al. Long-term follow-up of patients with Crohn's disease treated with azathioprine or 6-mercaptopurine. Lancet 1996;347:215-219.PMID: 8551879.


21. Jaspers GJ, Verkade HJ, Escher JC, de Ridder L, Taminiau JA, Rings EH. Azathioprine maintains first remission in newly diagnosed pediatric Crohn's disease. Inflamm Bowel Dis 2006;12:831-836.PMID: 16954801.


22. Candy S, Wright J, Gerber M, Adams G, Gerig M, Goodman R. A controlled double blind study of azathioprine in the management of Crohn's disease. Gut 1995;37:674-678.PMID: 8549944.



23. Kwak MS, Kim DH, Park SJ, et al. Efficacy of early immunomodulator therapy on the outcomes of Crohn's disease. BMC Gastroenterol 2014;14:85PMID: 24886458.



24. Cosnes J, Bourrier A, Laharie D, et al. Early administration of azathioprine vs conventional management of Crohn's Disease: a randomized controlled trial. Gastroenterology 2013;145:758-765.PMID: 23644079.


25. D'Haens G, Baert F, van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet 2008;371:660-667.PMID: 18295023.


26. Peyrin-Biroulet L, Deltenre P, Ardizzone S, et al. Azathioprine and 6-mercaptopurine for the prevention of postoperative recurrence in Crohn's disease: a meta-analysis. Am J Gastroenterol 2009;104:2089-2096.PMID: 19568226.



27. Golovics PA, Lakatos PL, Dávid G, et al. The effect of early immunosuppressive therapy on the rate of resections performed in patients with Chron's disease, in Veszprém county, Hungary, a population-based cohort study. Orv Hetil 2012;153:541-552.PMID: 22450143.


28. Lennard-Jones JE, Stalder GA. Prognosis after resection of chronic regional ileitis. Gut 1967;8:332-336.PMID: 6039720.



29. Hellers G. Crohn's disease in Stockholm county 1955-1974. A study of epidemiology, results of surgical treatment and long-term prognosis. Acta Chir Scand Suppl 1979;490:1-84.PMID: 293116.

30. López-Martín C, Chaparro M, Espinosa L, Bejerano A, Maté J, Gisbert JP. Adverse events of thiopurine immunomodulators in patients with inflammatory bowel disease. Gastroenterol Hepatol 2011;34:385-392.PMID: 21616565.


31. Lichtenstein GR, Abreu MT, Cohen R, Tremaine W. American Gastroenterological Association. American Gastroenterological Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology 2006;130:935-939.PMID: 16530531.


32. Kubota T, Nishida A, Takeuchi K, et al. Frequency distribution of thiopurine S-methyltransferase activity in red blood cells of a healthy Japanese population. Ther Drug Monit 2004;26:319-321.PMID: 15167635.


Fig. 1
The overall cumulative relapse rate in 47 biologic-naive CD patients. The overall cumulative relapse rate was 35.4% at 85.0 mo, on the basis of Kaplan-Meier analysis.
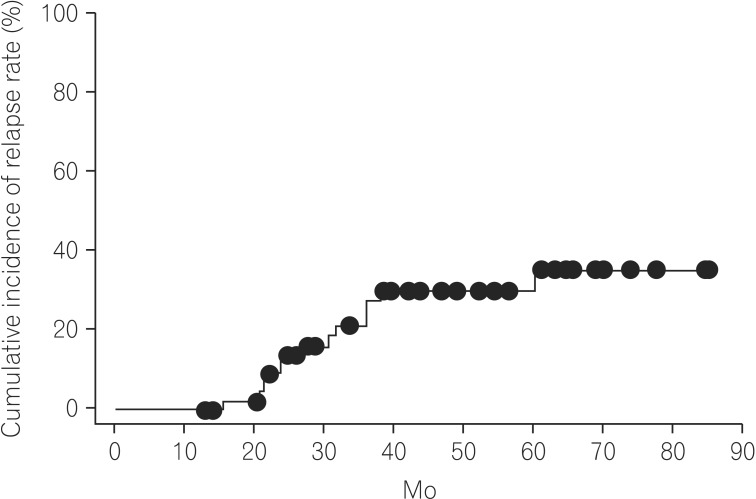
Fig. 2
The cumulative relapse rates in biologic-naive CD patients with and those without a history of surgery. The cumulative relapse rate in CD patients without a history of surgery was 27.2% at 85.0 mo (solid line), and that in those with such a history was 52.9% at 77.6 mo (dashed line) (P=0.071).

Fig. 3
In biologic-naive CD patients without a history of surgery, the cumulative relapse rates of patients with and those without digestive complications were evaluated. The 33 biologic-naive CD patients without a history of surgery were classified as follows: B1 (non-stricturing and non-penetrating) and B2+B3 (stricturing and penetrating) patients. The cumulative relapse rate in the B1 group was 11.1% at 85.0 mo (solid line) and that in the B2+B3 group was 58.5% at 69.0 mo (dashed line) (P=0.036).