Ulcerative colitis (UC) is an inflammatory bowel disease (IBD) characterized by chronic colon inflammation. The medications for UC include 5-aminosalicylic acid, corticosteroids, immunomodulators, and biologic agents [
1]. The latter two have been developing rapidly, and novel anti-tumor necrosis factor (TNF)-╬▒ agents have greatly improved outcomes for UC treatment refractory to conventional therapies. Other than biologic agents, tofacitinib (TOF) targets and blocks the Janus kinase pathway, a central hub in the cytokine network implicated in the modulation of immune and inflammatory responses [
2]. TOF has been effective for moderately to severely active UC in clinical trials [
3-
5], leading to its approval for UC treatment in 2018. However, real-world data from the clinical TOF application remains limited, especially regarding its clinical efficacy and safety. Herein, we present a short report on the real-world application of TOF on patients with refractory UC in our IBD center, focusing on long-term clinical efficacy and safety profiles.
We designed a retrospective observational study at the Tokyo Medical and Dental University (TMDU) hospital on Japanese patients under the care of board-certified gastroenterologists. We collected data on demographics, disease types, duration and activity, and treatment histories from patients with UC who had started TOF treatments between May and November 2018. The observation periods lasted up to 52 weeks after TOF administration. Patients were refractory to multiple treatments, such as corticosteroids, biologic agents, and a calcineurin inhibitor. TOF was initiated at 20 mg a day and decreasing to 10 mg a day after 8 weeks or more. All immunesuppressive agents, such as corticosteroids, immunomodulators, biologic agents, and calcineurin inhibitors, were withdrawn before starting TOF treatment. Disease activity was evaluated using the partial Mayo score (pMS), and laboratory data were collected at 4, 8, 12, 24, and 52 weeks after TOF administration. Our primary outcome was the clinical remission rate at 52 weeks; we also analyzed treatment efficacy and adverse events. We defined remission as pMS Ōēż 2 without individual subscores > 1, response as a decrease of pMS by at least 2, and meeting one of the following conditions: (1) a decrease of the bleeding subscore by at least 1; (2) bleeding subscore < 1; or (3) above-defined remission induced [
6,
7]. Failure was defined as TOF discontinuation due to symptom recurrence in patients in remission, nonresponse to TOF treatment, and serious adverse events [
8]. Differences in medians between the groups were compared using a non-parametric (Mann-Whitney), and comparisons between categorical variables were performed using chi-square test. TOF discontinuation-free survival was calculated. Kaplan-Meier curves were drawn for each subgroup and compared with log-rank (Mantel-Cox) test. SPSS version 26 (IBM Corp., Armonk, NY, USA) was used for statistical analysis, and the significant threshold was 0.05. The TMDU Institutional Review Board approved the study (IRB No. M2018-059). Informed consent was waived.
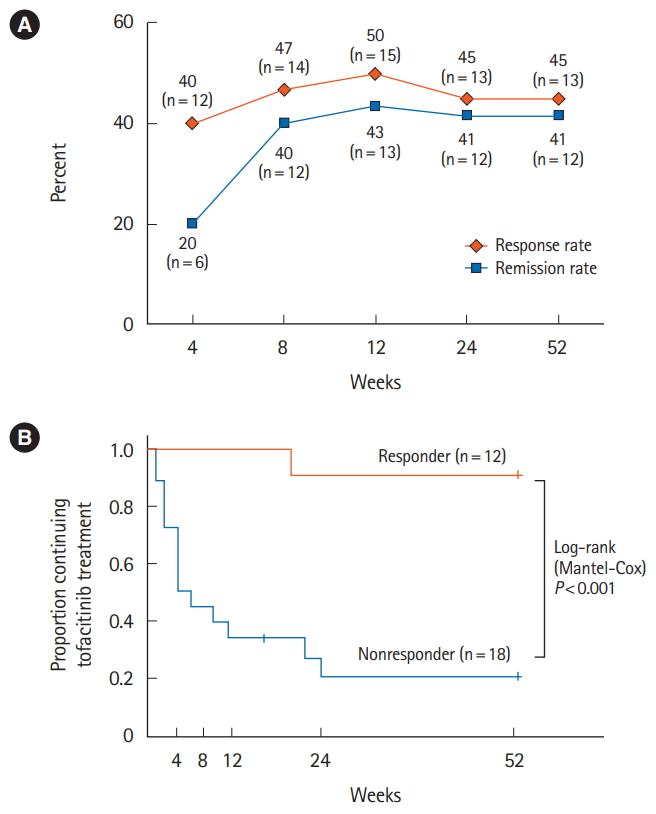
We enrolled 16 women (53.3%) and 14 men (46.7%) with a median age of 40.5 years (range, 28.3-60.0 years) and a median disease duration of 9.8 years (range, 6.5-11.8 years). There were 18 patients (60.0%) with extensive colitis and 12 (40.0%) with left sided UC. In addition, 29 patients (96.7%) showed moderate disease activity and 1 patient (3.3%) showed mild UC, comprising a mean pMS of 6 points (range, 3-7 points). Most patients were corticosteroid refractory (46.7%) or dependent (46.7%) and unresponsive to one or more anti-TNF-╬▒ agents (96.7%). Moreover, 36.6% patients were unresponsive to a calcineurin inhibitor (Table1). Patients were monitored for up to 52 weeks (14 patients were monitored until 52 weeks), and the median follow-up period was 23.0 weeks (range, 4.0-52.0 weeks). At 4 weeks of TOF treatment, 12 patients (40.0%) responded to the medication, and 6 (20.0%) achieved remission. Both the response and remission rates continued to exceed 40% from 8 to 52 weeks of TOF treatment (
Fig. 1A). At 52 weeks, 12 patients were in remission, 1 patient who had achieved remission experienced slight worsening without requiring TOF discontinuation, and 16 patients experienced treatment failure, of which 13 were nonresponsive and 3 developed recurrence. At 52 weeks, the responders showed significantly improved pMS from as early as 4 weeks after TOF administration, which continued to improve until 52 weeks. Overall, 91.7% (95% confidence interval [CI], 83.7%-99.7%) TOF responders at 4 weeks were able to continue the treatment for 52 weeks. However, 80.0% (95% CI, 70.1%-89.9%) of TOF nonresponders at 4 weeks discontinued TOF treatment due to lack of efficacy (
Fig. 1B). The background characteristics of TOF responders and nonresponders at 4 weeks were similar without any significant difference, except in terms of previous calcineurin inhibitor failure (
Table 2). For the safety evaluation, the most common adverse events were non-serious infections (n = 9, 30.0%). Three patients (10.0%) presenting herpes zoster infections were treated successfully with anti-viral medication. One patient, a 60-year-old male using 20 mg TOF, developed herpes zoster at 16 weeks, and others, a 67-year-old male and a 40-year-old female using 10 mg TOF, developed herpes zoster at 24-25 weeks; all patients presented a history of previous corticosteroid exposure, although not at the time of infection. Also, 4 patients (13.3%) started medication for dyslipidemia. No serious adverse events were recorded, and no adverse events resulted in discontinuation of TOF treatment (
Supplementary Table 1).
In here, we present a preliminary report on the clinical efficacy and safety of TOF treatment for refractory UC, a treatment at an early stage of real-world application. Although the full breadth of the clinical implications and safety profiles of this treatment remain unclear, Lair-Mehiri et al. [
9] have just reported on real-world experience with TOF, in which the efficacy of TOF was > 30% at 48 weeks and more than half of the patients could continue TOF treatment for 48 weeks, avoiding colectomy. We showed consistent efficacy of TOF in that 40.0% patients were responsive and 20.0% in remission at 4 weeks, and both rates continued to exceed 40% from 8 to 52 weeks. LairMehiri et al. [
9] have also highlighted the safety profile of TOF, stating that it is safe enough for outpatient use, and our data hopefully supports their data. Although, we encountered non-serious adverse events associated with TOF treatment, some serious adverse events have been reported in TOF users with rheumatoid arthritis since 2012 [
10]. It is not the same case for patients with UC as their background is different from that of patients with rheumatoid arthritis. However, patients with IBD are at a certain risk of deep vein thrombosis; thus, it is very important to account for cardiovascular risks when using TOF for patients with UC and further extensive analyses are needed. Our data showed that, at 4 weeks, most TOF responders could continue the treatment for 52 weeks, exhibiting a continued positive response with no serious adverse events. This suggests that predicting long-term TOF efficacy after 4 weeks of treatment is possible, allowing for a quick and efficient clinical decision as to whether continue or discontinue the TOF treatment. We are aware that our small sample cohort, distinct patient population, 1-year of follow-up, and single center evaluation represent substantial study limitations, but our report provides new information on real-world TOF treatment for patients with refractory UC.