|
 |
- Search
| Intest Res > Volume 18(3); 2020 > Article |
|
Abstract
NOTES
FINANCIAL SUPPORT
The authors received no financial support for the research, authorship, and/or publication of this article.
CONFLICT OF INTEREST
Matsuoka K received lecture fees from Mitsubishi Tanabe Pharma, Takeda Pharmaceutical, Janssen Pharmaceutical, Abbvie, EA Pharma, Pfizer, Mochida Pharmaceutical, Kyorin Pharmaceutical, Zeria Pharmaceutical, Kissei Pharmaceutical, Nippon Kayaku, Thermo Fisher Scientific, Alfresa Pharma, JIMRO, Miyarisan Pharmaceutical; consultancy fees from Thermo Fisher Scientific, Alfresa Pharma, EA Pharma, Takeda Pharmaceutical, Janssen, Abbvie, Mitsubishi Tanabe Pharma; research grants from Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, Abbvie, Takeda Pharmaceutical, Pfizer, Nippon Kayaku, Shionogi Pharmaceutical, EA Pharma, Zeria Pharmaceutical, Kissei Pharmaceutical.
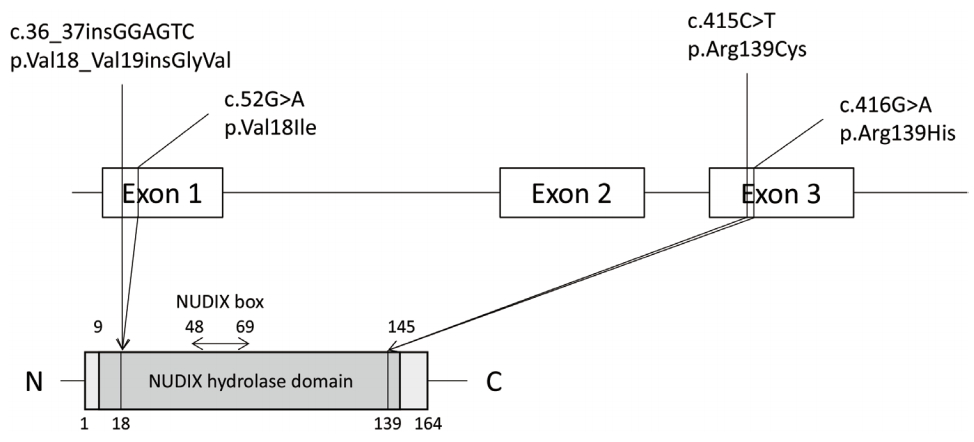
Fig. 1.
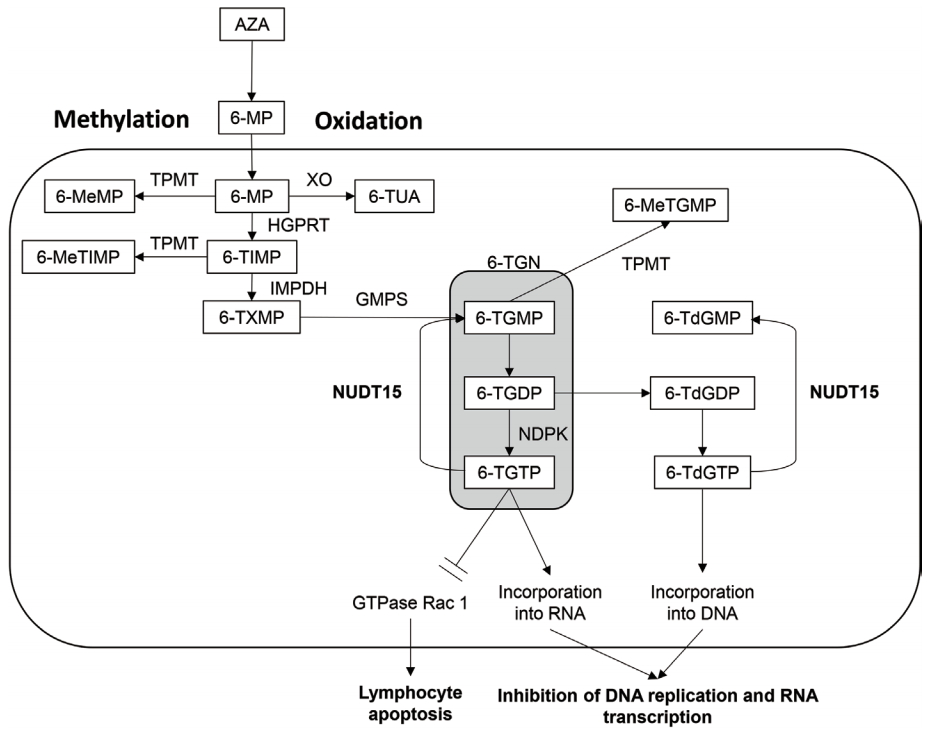
Table 1.
| Author | Country | No. | C/C | C/T | T/T |
|---|---|---|---|---|---|
| Chang et al. [28] | Korea | 145 | 105 (72.4) | 38 (26.2) | 2 (1.4) |
| Lee et al. [29] | Korea | CD 165 | 106 (64.2) | 28 (17.0) | 6 (3.6) |
| Asada et al. [9] | Japan | 264 | 213 (80.7) | 48 (18.2) | 2 (1.1) |
| UC 89 | |||||
| CD 72 | |||||
| HC 103 | |||||
| Kakuta et al. [30] | Japan | 135 | 107 (79.3) | 23 (17.0) | 5 (3.7) |
| CD 111 | |||||
| UC 23 | |||||
| IBDU 1 | |||||
| Sato et al. [31] | Japan | 160 | 117 (73.1) | 35 (21.9) | 8 (5.0) |
| CD 86 | |||||
| UC 72 | |||||
| IBDU 1 | |||||
| BD 1 | |||||
| Chao et al. [32] | China | 732 | 557 (76.1) | 164 (22.4) | 11 (1.5) |
| CD 660 | |||||
| UC 60 | |||||
| IBDU 12 | |||||
| Sutiman et al. [33] | Singapore | 129 | 111 (86.0) | 16 (12.4) | 2 (1.6) |
| Chinese (65.1%) | CD 89 | ||||
| Malay (9.3%) | UC 40 | ||||
| Indian (24.0%) | |||||
| Others (1.6%) | |||||
| Shah et al. [34] | India | 69 | 60 (87.0) | 8 (11.6) | 1 (1.4) |
| UC 34 | |||||
| CD 24 | |||||
| AIH 11 |
Table 2.
Table 3.
| Study | C/C | C/T | T/T |
|---|---|---|---|
| Kakuta et al. [30] | 1/106 (0.9) | 4/19 (17.4) | 5/5 (100) |
| Lee et al. [29] | 0.8 | 24.2 | 100 |
| Yang et al. [16] | 7/788 (0.9) | 45/176 (25.6) | 14/14 (100) |
| Asada et al. [9] | 2/127 (1.6) | 2/32 (6.3) | 2/2 (100) |
| Sato et al. [31] | 3/109 (2.8) | 8/33 (24.2) | 5/7 (71.4) |
REFERENCES
- TOOLS