|
 |
- Search
| Intest Res > Volume 18(3); 2020 > Article |
|
Abstract
Background/Aims
Methods
Results
Conclusions
NOTES
FINANCIAL SUPPORT
The authors received no financial support for the research, authorship, and/or publication of this article.
CONFLICT OF INTEREST
Bessissow T is a speaker or advisory board for Janssen, AbbVie, Takeda, Pfizer, Shire, Ferring, PendoPharm, Merck. He was supported by unrestricted research grants from Janssen, Abbvie, Pentax, and Echosense. But, all authors had no conflicts of interests to declare pertaining to submission.
AUTHOR CONTRIBUTION
Conceptualization: Alrajhi S, Afif W. Methodology: Alrajhi S, Germain P, Martel M, Afif W. Formal analysis: Alrajhi S, Germain P, Martel M. Project administration: Alrajhi S, Afif W. Visualization: Alrajhi S, Germain P, Martel M, Afif W. Writing - original draft: Alrajhi S, Germain P, Martel M, Afif W. Writing - review and editing: Alrajhi S, Germain P, Martel M, Lakatos P, Bessissow T, Al-Taweel T, Afif W. Approval of final manuscript: all authors.
ACKNOWLEDGEMENTS
SUPPLEMENTARY MATERIALS
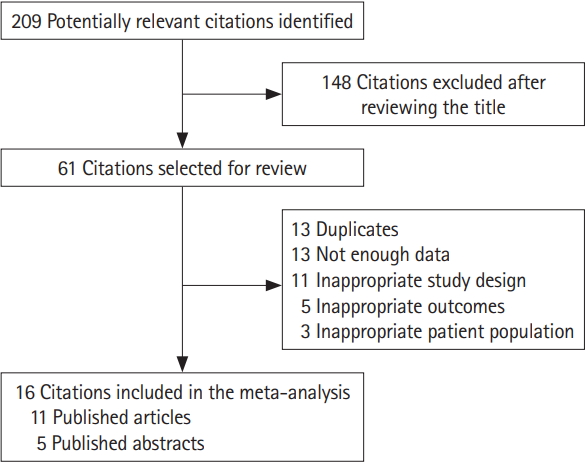
Fig. 1.

Fig. 3.
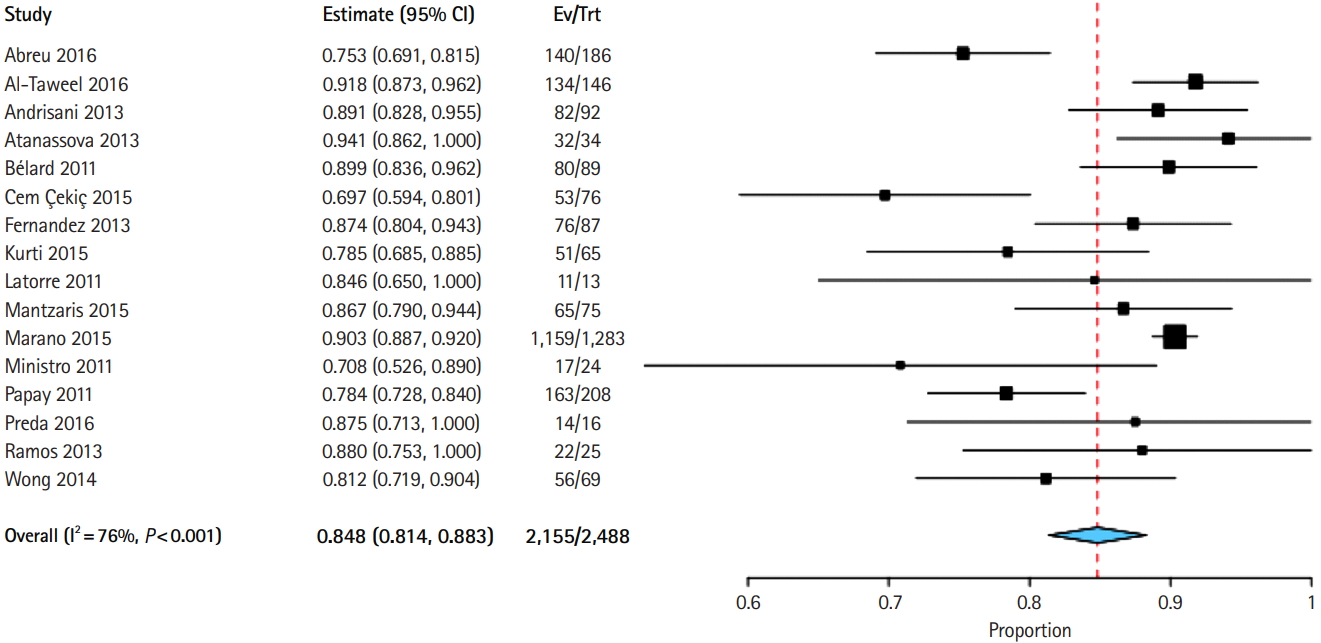
Fig. 4.
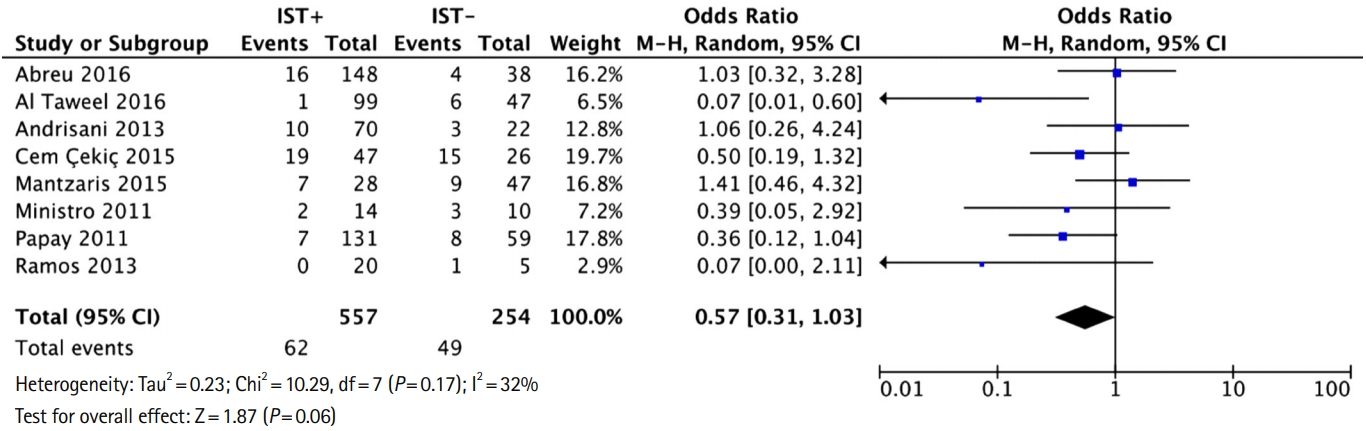
Fig. 5.
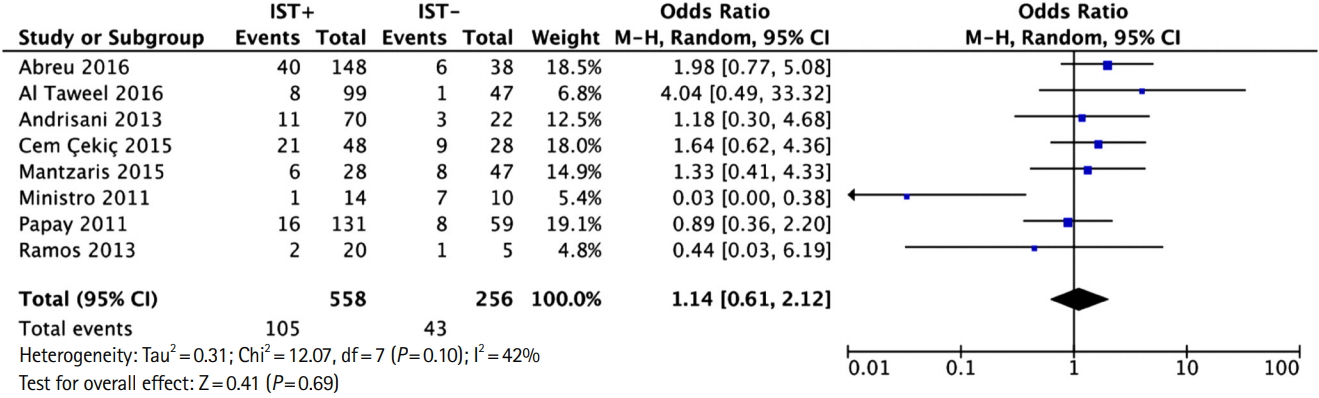
Table 1.
| Study | Year | Country | 2017 WHO estimated TB incidence (thousand) | Study design | No. | Age (yr) | BCG vaccinated (%) | PPD cutoff (mm) |
|---|---|---|---|---|---|---|---|---|
| Abreu et al. [19] | 2016 | Portugal | 1.8 | Prospective observational cohort | 186 | NR | 100 | 5 |
| Al-Taweel et al. [20] | 2018 | Canada | 1.8 | Prospective observational cohort | 146 | Mean 39.6 | 16.4 | 5 If on IST, 10 if high risk population, 15 if no risk |
| Andrisani et al. [21] | 2013 | Italy | 3.8 | Prospective observational cohort | 92 | Range 36-43 | 1.0 | 5 If on IST,10 others |
| Atanassova [13]a | 2013 | Bulgaria | 1.5 | Prospective observational cohort | 34 | Mean 39 | 100 | NR |
| Bélard et al. [22] | 2011 | Denmark | 0.26 | Prospective observational cohort | 89 | Range 18-84 | 33.7 | 5 (BCG-), 10 (BCG+) |
| Çekiç et al. [23] | 2015 | Turkey | 12 | Retrospective observational cohort | 76 | Mean 42 | NR | 5 |
| Fernandez et al. [14]a | 2013 | Spain | 4.2 | Prospective observational cohort | 87 | Mean 40 | 23.0 | NR |
| Kurti et al. [12] | 2015 | Hungary | 0.65 | Prospective observational cohort | 65 | Mean 24 | 100 | 5 |
| Latorre et al. [15]a | 2011 | Spain | 4.2 | Prospective observational cohort | 13 | NR | NR | NR |
| Mantzaris et al. [24] | 2015 | Greece | 0.41 | Prospective observational cohort | 75 | Mean 37 | 34.7 | 5 If IST, 10 others |
| Marano et al. [16]a | 2015 | USA | 9.1 | Prospective randomized control trial | 1,283 | Mean 40 | 39.0 | 5 |
| Ministro et al. [17]a | 2011 | Portugal | 1.8 | Prospective observational cohort | 24 | NR | 100 | 5 |
| Papay et al. [25]a | 2011 | Austria | 0.57 | Prospective observational cohort | 208 | Mean 37 | 100 | 5 If IST, 10 others |
| Preda et al. [18]b | 2016 | Romania | 13 | Retrospective observational cohort | 16 | Mean 38 | NR | 5 |
| Ramos et al. [26] | 2013 | Spain | 4.2 | Prospective observational cohort | 25 | Median 32 | 8.0 | 5 |
| Wong et al. [11] | 2014 | China | 790 | Prospective observational cohort | 69 | NR | 73.0 | 5 |