Infliximab biosimilar CT-P13 is interchangeable with its originator for patients with inflammatory bowel disease in real world practice
Article information
Abstract
Background/Aims
An interim analysis of post-marketing surveillance of CT-P13, an infliximab biosimilar, was performed to evaluate its safety and efficacy in Japanese patients with inflammatory bowel disease.
Methods
Patients were prospectively enrolled between November 2014 and March 2017, after the launch of CT-P13 in Japan, and case report forms of patients followed for at least 4 months were analyzed as of July 2018.
Results
Of 523 patients in the analysis set, 372 remained on CT-P13 therapy, while 54 (20.2%) of 267 patients with Crohn’s disease, and 97 (37.9%) of 256 patients with ulcerative colitis were withdrawn during follow-up. A total of 144 adverse drug reactions (ADRs) were reported in 106 patients (20.3%). Infusion reaction was the most frequent ADR observed in 49 patients (9.4%). Efficacy parameters decreased immediately after the start of treatment in naïve patients to anti-tumor necrosis factor-α antibody. In the patients switched from originator infliximab for nonmedical reasons, the decreased parameters due to proceeded treatment with the originator were maintained in low ranges, and the treatment continuation rate was high with low ADR incidence. In contrast, in patients switched for medical reasons such as adverse event or loss of response, the incidence of ADRs was high. However, the efficacy parameters were improved, and the treatment continuation rate was not significantly different from that of the naïve patient group.
Conclusions
In this interim analysis, CT-P13 was comparable to the originator infliximab with respect to ADRs and efficacy, and is therefore considered to be a cost-efficient interchangeable biosimilar for Japanese patients with inflammatory bowel disease.
INTRODUCTION
Infliximab (IFX), which was first approved in the United States in 1998, is widely used for treatment of various inflammatory diseases, including rheumatoid arthritis (RA) and IBD. CT-P13 is the first antibody biosimilar, with the same amino acid sequence and higher order structure as its originator IFX. It binds to the inflammatory cytokine, TNF-α, and exhibits anti-inflammatory activity [1,2]. After expiration of patents and data exclusivity period of the originator IFX, the biosimilar CT-P13 was developed for socioeconomic reasons.
Crossover pharmacokinetics, pharmacodynamics, and multicenter randomized phase 3 studies of CT-P13 were conducted with patients with ankylosing spondylitis and RA. Clinical comparability to the originator IFX was demonstrated with respect to adverse drug reaction (ADR) profile as well as efficacy for those diseases [3,4]. In Japan, CT-P13 was approved based on similar results in clinical pharmacokinetics/pharmacodynamics and comparative phase 1/2 studies conducted with Japanese RA patients [5], in accordance with “Policies for the Assurance of the Quality, Safety and Efficacy of Biosimilar Products” (2009). However, CT-P13 had not been thoroughly investigated with Japanese patients, and no clinical study had been conducted in patients with IBD. Therefore, Nippon Kayaku Co., Ltd. (Tokyo, Japan) initiated post-marketing surveillance (PMS) of Japanese IBD patients in 2014, in response to requests from the Japanese regulatory authority. Seven hundred patients were enrolled in the PMS, and an interim analysis of the finalized data from 523 patients, followed up at least 4 months, was conducted. While CT-P13 is not widely used in Japan like some European countries because of differences in health insurance and drug pricing systems, this interim analysis of the PMS will affect the clinical use of CT-P13 by showing its comparability to and interchangeability with the originator in clinical practice.
METHODS
1. Patients
Japanese patients with IBD were prospectively enrolled in the PMS during a 28-month enrollment period starting in November 2014. The target number for analysis was 300 patients, who were treated at least for 4 months, including 100 patients with CD and 100 with UC. This sample size provides a power of 95% or more to detect 1 patient reporting an adverse event (AE) at an incidence of 1%.
The PMS was required by the Ministry of Health, Labour and Welfare (MHLW) as a condition for approval of CT-P13, and conducted as a centralized registration system of all-case surveillance. The protocol of PMS was approved by the MHLW, and all PMS procedures complied with the Good Post-Marketing Study Practice Ordinance of MHLW. Therefore, formal approval by an ethics committee was not required.
2. Treatment
The approved dosage of CT-P13 is 5 mg/kg on weeks 0, 2, and 6, and every 8 weeks thereafter. Dose escalation up to 10 mg/kg and/or interval reduction down to 4 weeks is allowed for CD patients with diminished response after week 6. In the PMS, therefore, naïve patients initially received induction therapy at a dose of 5 mg/kg, the patients switched for nonmedical reasons received their first dose of biosimilar during maintenance therapy at intervals of 8 weeks, and some CD patients received higher-dose treatment.
3. Surveillance Methods
The duration of follow-up was 2 years, and case report forms (CRFs) were collected from each patient 4 months, 1 year, and 2 years after the start of treatment. In addition to patient characteristics and disease status, CT-P13 regimen, previous therapy, concomitant drugs, and ADR-related information, including subjective/objective findings and laboratory test data, were collected. Reported ADRs were coded by System Organ Class and Preferred Term, as listed in the Medical Dictionary for Regulatory Activities (MedDRA/J; version 20.0). Incidences of increased ALT and ALP were evaluated in accordance with the diagnostic criteria for drug-induced liver injury set by the Japan Society of Hepatology. Specifically, ALT more than twice the upper limit of normal (ULN), and ALP more than the ULN at each clinical site, were defined as increased ALT and ALP, respectively. When baseline values exceeded the ULN, the increase in ALT or ALP during CT-P13 treatment was used for this definition.
Efficacy was periodically assessed by the physician as effective, partially effective, ineffective, progressed, or not evaluable. The disease status evaluated by endoscopic examination was reported as severe, moderate, mild, or in remission. In addition to physicians’ assessments, changes in CDAI, partial Mayo score, and CRP were used as efficacy parameters. The data obtained on the nearest day to the scheduled administration time points (on weeks 2, 6, and 14, and every 8 weeks thereafter for naïve patients and patients switched for medical reasons; and every 8 weeks for patients switched for nonmedical reasons) were used as the representative values.
The response of each CD patient to CT-P13 was evaluated by comparing the CDAI on week 14 to baseline, in naïve patients and those switched for medical reasons; and CDAI on week 16 to baseline, in those switched for nonmedical reasons. Responders were defined as showing decreases of more than 70 from baseline, or scores less than 150 in patients with baseline scores above 150. Consistent responders were defined as having scores less than 175 throughout the relevant period, or showing increases of less than 70 in patients with baseline scores below 150. Nonresponders were defined as showing score increases from below 150 at baseline to above 175, or having scores above 150 throughout the relevant period without decreases of 70 or more. Partial Mayo scores were employed for the evaluation of each UC patient in the same manner, with a cutoff score of 3.
4. Database Search
Clinical data, including ALT and ALP, for patients prescribed IFX during the same period as the PMS were obtained from EBM Provider, a Japanese hospital-based administrative database created by Medical Data Vision Co., Ltd. (Tokyo, Japan), as reference data for comparison with CT-P13 [6]. Incidences of increases in ALT/ALP were assessed using the common standard laboratory values proposed by the Japanese Committee for Clinical Laboratory Standards.
5. Statistical Analysis
To identify risk factors that affect the occurrence of ADR and infusion reaction (IR), patient factors with P<0.2 in the Fisher exact probability test or the chi-square test were selected in a stepwise manner to conduct multivariate analysis using a logistic regression model.
The duration of treatment was plotted by the Kaplan-Meier method, with discontinuation of treatment as an event. Patients with whom treatment was discontinued within 7 days after the start of treatment were excluded. Differences between patient groups were analyzed using the generalized Wilcoxon test.
RESULTS
1. Patient Characteristics
In order to investigate the safety and efficacy of a biosimilar of IFX, we initiated PMS, and registered 700 patients during the enrollment period. In this interim analysis, 523 patients whose CRFs at month 4 had been collected were included in the safety and efficacy analysis set (Fig. 1A). Further reported data in 270 collected CRFs at 1 year and in 56 final CRFs at the end of 2-year follow-up period were fixed and analyzed. As shown in Fig. 1B, the ratios of biologic-naïve patients to patients switched for nonmedical reasons from the originator IFX were quite different between the CD and UC groups. The number of patients switched to CT-P13 for medical reasons was less than 20% in both CD and UC patients. This patient group with medical switch consisted of (a) patients switched from the originator IFX to CT-P13 due to medical reasons such as AEs or loss of response, (b) patients received CT-P13 after adalimumab (ADA), and (c) patients retreated with CT-P13 for relapse after discontinuation of IFX due to remission of disease.
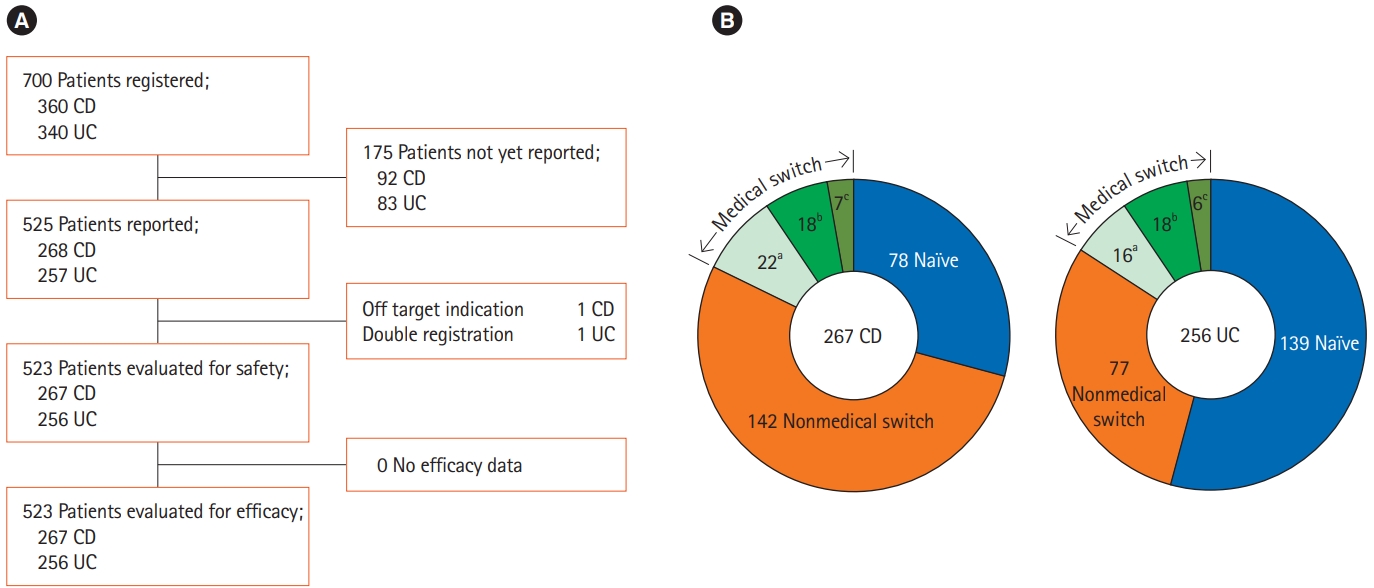
Patient demography (A) and patient classification based on prior biologic therapy and reasons for switching to CT-P13 (B). Patients were classified into 3 groups: naïve patients who had no previous anti-TNF-α antibody treatment, patients switched for nonmedical reasons such as institutional policy or economic reason (nonmedical switch), and patients switched for medical reasons (medical switch). The medical switch group was further devided into 3 subgroups. aSwitching to CT-P13 from infliximab (IFX) due to adverse events or insufficient efficacy; bSwitching from adalimumab; cRetreatment with CT-P13 for relapse after discontinuation of IFX due to remission of disease.
The characteristics of patients and the disease statuses in these groups of patients with CD or UC were markedly different (Table 1). The duration of disease in naïve patients was shortest, and most of the naïve patients had severe or moderate disease. Patients who were switched for nonmedical reasons showed stable disease conditions, as indicated by decreased CDAI or partial Mayo scores due to previous treatment with IFX. They included lower proportions of patients with external fistulas or anal lesions in CD patients, and with steroid dependence in UC patients. The patients in the medical switch group had the longest disease duration, but the disease severity was comparable to that in the biologic-naïve patient group. Concomitant use of steroids was less frequent in the nonmedical switch group, and the rate was higher in UC patients than CD patients. The proportions of patients concomitantly administered immunomodulators showed no clear differences between the patient groups.
2. Treatment Status
The median initial dose of CT-P13 in this PMS was 5 mg/kg, which was same as the approved standard dose. While almost all patients with UC were administered the usual dosage less than 8 mg/kg, higher doses were used in 61 patients (22.8%) with CD, for whom dosage up to 10 mg/kg is permitted when response become low. However, the incidences of ADRs and IR with higher dose of CT-P13 were not high as compared with lower standard doses, and no serious ADR was reported.
The range of infusion duration was 60 to 600 minutes, and more than 50% of patients received 120-minute infusion. The incidences of ADR and IR were independent of the infusion duration.
3. Incidence of ADRs
Of the 523 patients in the safety analysis set, a total of 144 ADRs were reported in 106 patients (20.27% by number of patients, 26.64% per 100 person-years), and the most common ADR was IR reported in 49 patients (9.37% and 12.31%), as listed by System Organ Class in Table 2.
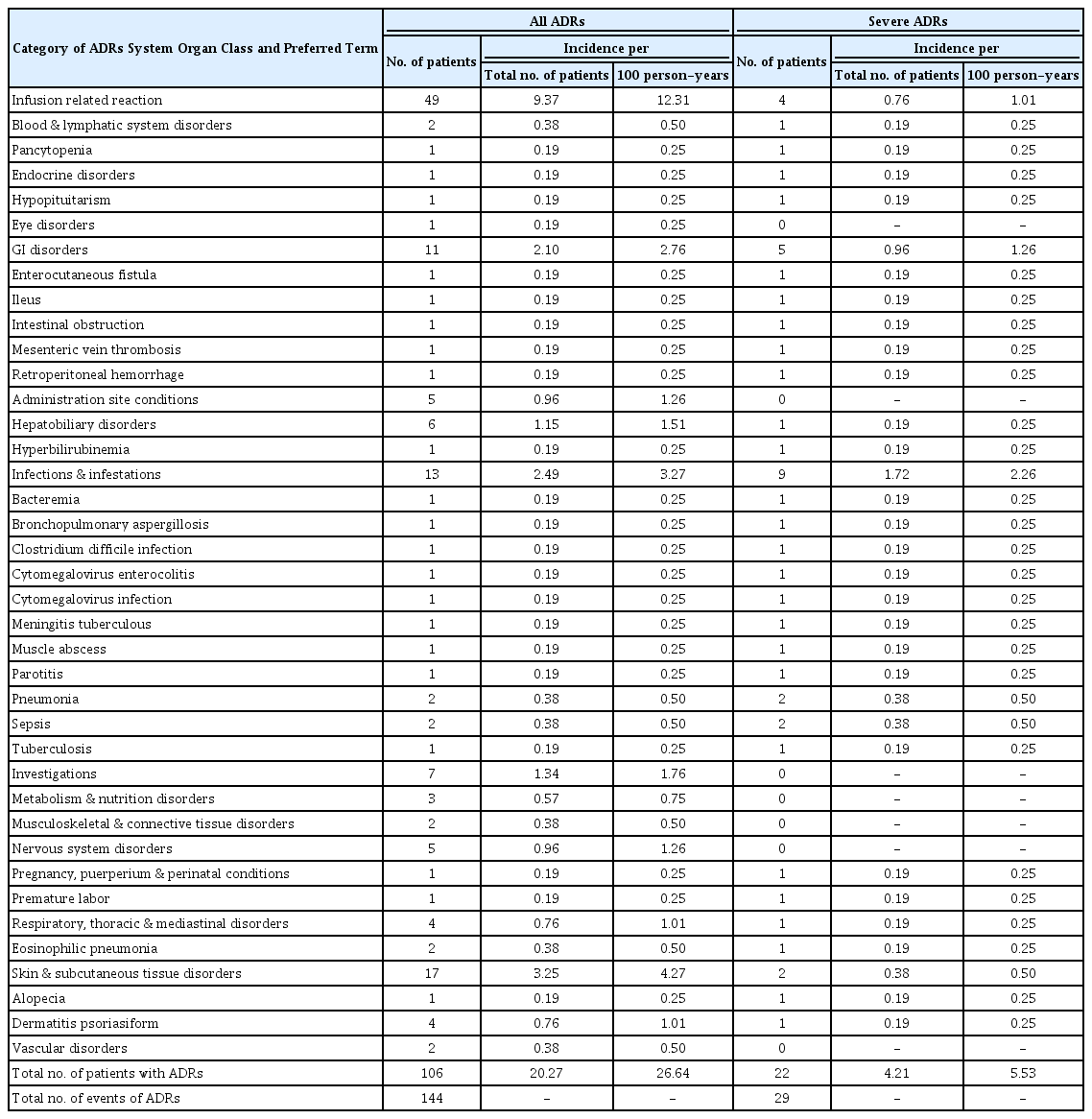
Incidence of ADRs and Severe ADRs to CT-P13 in 523 IBD Patients in the Safety Analysis Set during the Observation Period of 397.9 Person-Years
Twenty-nine serious ADRs were reported in 22 patients (4.21% and 5.53%). Although 20 patients recovered, 1 patient who experienced 3 serious ADRs, died of retroperitoneal hemorrhage and pneumonia following pancytopenia due to reactivation of cytomegalovirus. This patient showed a complicated clinical course after long-term use of steroid, and CT-P13 was not the sole cause for the severe ADRs, although involvement of CT-P13 cannot be ruled out. Recovery from the remaining one serious ADR of tuberculous meningitis could not be confirmed due to transfer to another hospital. A total of 35 patients, including these 2 patients, were withdrawn from CT-P13 therapy due to ADRs, of which 23 cases were IR.
Six cases of hepatobiliary disorders were reported by physicians. In addition, increased ALT and/or ALP fulfilling the diagnostic criteria for drug-induced liver injury set by the Japan Society of Hepatology was observed in 66 patients (16.3%). However, no patient discontinued CT-P13 treatment due to the increase in ALT/ALP, although 9 patients were withdrawn from treatment for other reasons such as IR, alopecia, insufficient efficacy, and personal reasons. The marker levels decreased to within normal range under treatment with CT-P13 in 45 patients (recovery in 12 patients was not confirmed, because subsequent CRFs were not collected), showing that the increases in liver function markers were transient, and did not become serious in any patient.
The incidences of ADRs and IRs in patient groups according to the prior biologics were compared in Table 3. In naïve patient group, ADRs were reported in 24.9% of patients, and the incidence of IRs was 9.7%. The incidences of ADRs and IRs were lower in patients who were switched for nonmedical reasons than other patient groups. On the contrary, ADRs were reported in 32.2% of the patients in medical switch group, and IRs was frequently observed in patients pretreated with originator IFX or ADA.
4. Risk Factors for ADRs
To investigate the impact of patient factors on ADRs, multivariate analysis was performed using a logistic regression model (Table 4). IR and other systemic ADRs were analyzed separately, since those had different characteristics as well as apparent timing after administration of CT-P13.

Multivariate Logistic Analysis of Incidence of Infusion Reaction and Other ADRs to CT-P13 Based on Patient Factors
History of drug allergy was the most significant factor for IR (P<0.01). A particularly high incidence of IR was reported in patients who had allergy to IFX (31.8%, 7/22). The incidence of IR in patients who were allergic to ADA or 5-aminosalicylic acid were 28.6% (2/7) and 20.8% (5/24), respectively. Patients who had a history of allergies to other drugs or nonmedicinal substances did not show higher incidence of IR compared to patients without an allergy history.
Prior treatment with biologics was another significant factor for IR. The incidence of IRs in biologic-naïve patients without prior biological therapy was significantly higher than in patients switched for nonmedical reasons (P<0.01), but also significantly lower than in patients switched for medical reasons (P<0.01).
For ADRs other than IR, only switching from prior IFX for nonmedical reasons was a significant factor lowering their incidence. No other factors were significant, but principal disease (CD or UC) and concurrent disease affected the incidence of other ADRs (P=0.074 and P=0.068, respectively).
5. Treatment Continuation
For evaluation of the general usefulness of CT-P13, the treatment durations of CT-P13 were analyzed by the Kaplan-Meier method with mean observation period of 299 days. The treatment continuation rate was higher in CD than UC patients (79.8% vs. 62.1%, P<0.001; generalized Wilcoxon test). Patient withdrawal due to ADRs was lower in CD patients (5.2%) than in UC patients (11.3%), as was withdrawal due to insufficient efficacy (11.1% and 15.6% in CD and UC patients, respectively).
For both CD and UC patients, the treatment duration was significantly longer in patients switched for nonmedical reasons than in naïve patients or patients switched for medical reasons (Fig. 2). Although treatment continuation rate was higher in the naïve group than in the medical reasons group over time, no significant difference was shown due to limited number of patients in each group.

Kaplan-Meier plot of treatment period of CT-P13 in CD (A) and UC patients (B). Patients who ceased further treatment with CT-P13 earlier than 7 days from the initial administration were excluded from the plot. Statistically significant differences were analyzed by the generalized Wilcoxon test. aP<0.05, bP<0.01, cP<0.001.
6. Efficacy Analysis
Next, we evaluated the efficacy of CT-P13 on the basis of clinical scores (CDAI and partial Mayo score) and CRP, as well as assessment by primary-care physicians (Fig. 3). In patients switched for nonmedical reasons, lowered clinical scores and CRP by the previous treatment with the originator were maintained after switching to CT-P13, and the efficacy rate assessed by physicians to be “effective” or “partially effective” was maintained at 90% or more. In naïve patients, the effect of CT-P13 appeared immediately after initiation of treatment, as shown by decrease in CRP on week 2, followed by decreases in CDAI or partial Mayo score. These efficacy parameters remained in low ranges thereafter. In patients switched from original biologics for medical reasons, all the variables decreased, as seen in naïve patients, and the efficacy rate assessed by physicians increased and remained above 80%.
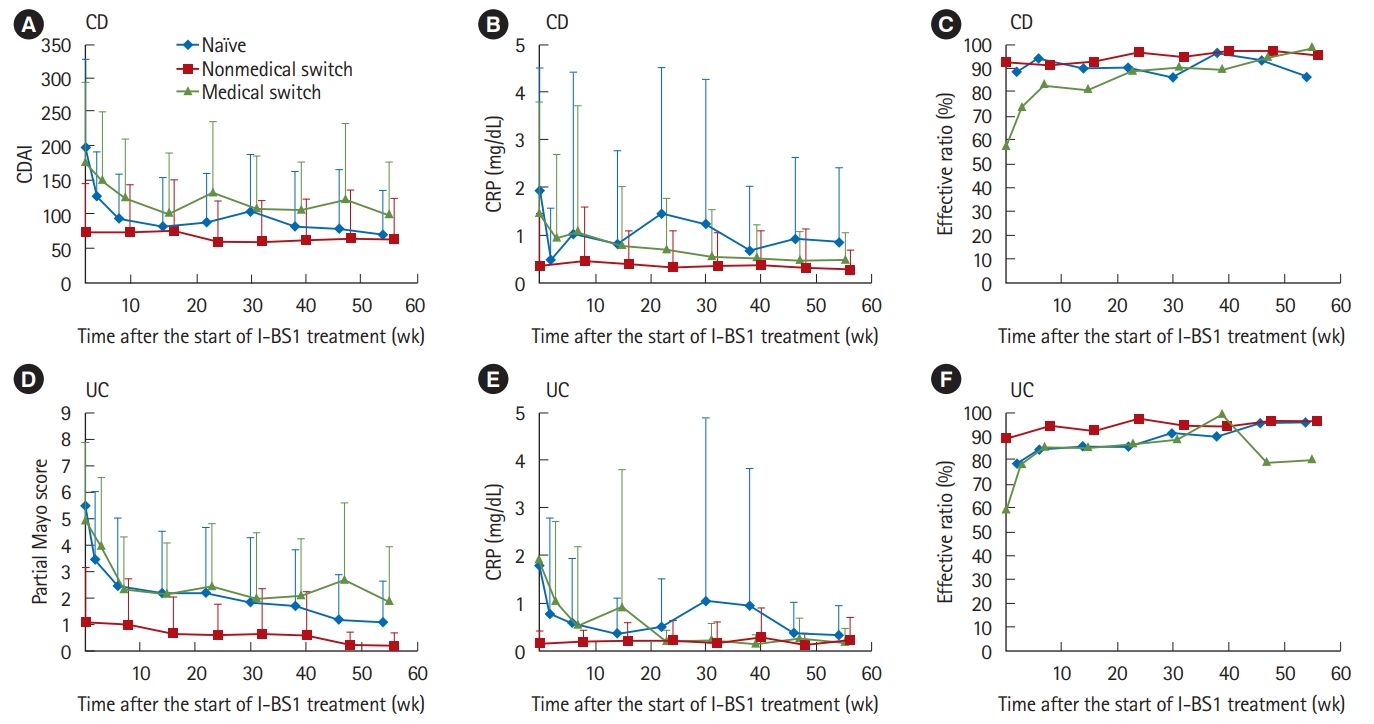
Changes in 3 efficacy variables in CD (A-C) and UC patients (D-F). Evaluated CDAI (A), partial Mayo score (D), and measured CRP (B, E) are expressed as mean±SD. Effective rate of clinician-reported efficacy (C, F) is based on assessment of the response to CT-P13 as effective or partially effective by primary care physicians.
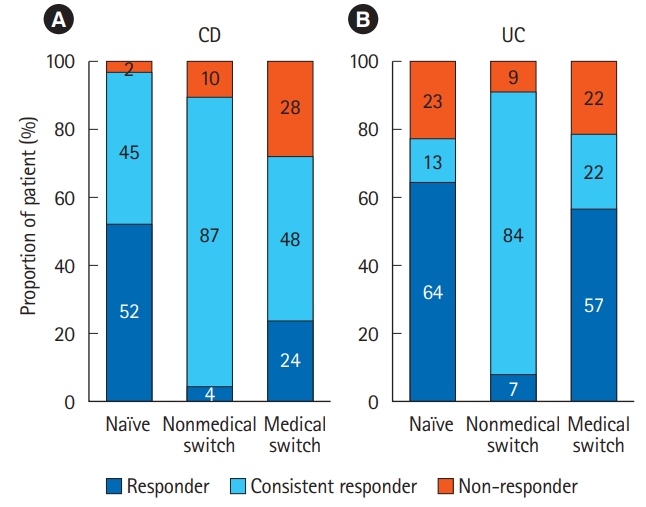
Furthermore, we evaluated responses of each patient to CT-P13 on week 14 or 16 based on changes in CDAI or partial Mayo score (Fig. 4). Most of naïve patients were assessed as responders showing substantial decreases from baseline clinical score. On the contrary, in the nonmedical switch group, most patients had low clinical scores even at the start of treatment with CT-P13 due to previous IFX therapy, and were classified as consistent responders with scores that remained low. In the medical switch group, a high proportion of nonresponders showed increases from low baseline scores or no change from high baseline scores, but responders and consistent responders still made up the majority of patients (72% and 79% for CD and UC, respectively). The proportions of responders and consistent responders were higher in patients switched from ADA than in those switched from IFX (82.6% vs. 65.0%). The favorable response rate (77.8%) was observed in patients retreated with CT-P13 after discontinuation of originator IFX due to remission, as high efficacy rate was reported with IFX retreatment [7-9].
DISCUSSION
This is the first report on the ADR profile and efficacy outcome of treatment with CT-P13 in Japanese patients with IBD in real-world settings. There are inherent limitations to draw a definitive conclusion in PMS data such as sparsely collected data, insufficient reporting items, and non-comparative study design with the originator. However, the real-world information in a large-scale survey with 523 patients must be useful when CT-P13 is planned to use in clinical practice. Actually, the incidences of ADRs of CT-P13 in the biologic-naïve patients were shown to be comparable to those reported in the PMS of the originator IFX in Japanese IBD patients who were naïve to biologics (CD; 24.4% vs. 24.0% and UC; 28.1% vs. 18.5%) [10]. In addition to incidence, the types of ADRs reported with CT-P13 were similar to those reported with the originator, and the most common ADR was IR. Multivariate analysis of patient factors identified history of drug allergy and prior biologics as significant factors associated with a higher IR incidence. The drug of allergy history was mostly prior IFX, indicating the need for careful attention when switching in patients with a history of drug allergy to IFX. In fact, the incidence of ADRs, especially IRs, was higher in patients switched for medical reasons, and substitution to biosimilar from the originator IFX for AEs or loss of response was not recommended.
Elevated ALT and AST levels meeting the diagnostic criteria for drug-induced liver injury were also frequently observed (16.3%). This incidence with CT-P13 was similar to that observed in IFX-treated patients (17.4%) registered in the Japanese medical database created by Medical Data Vision. None of the patients discontinued treatment with CT-P13, and liver enzyme markers decreased from elevated levels to their normal ranges over the course of treatment, as previously reported on IFX-induced liver dysfunction [11,12]. The occurrence of liver injury was not affected by concomitant use of immunomodulators or steroids, but correlated with disease severity.
CT-P13 significantly decreased CDAI, partial Mayo score, and CRP, and kept their values low over time. The effectiveness of CT-P13 was also confirmed by evaluation of endoscopic mucosal healing, which has been recently recognized as an important therapeutic target for IBD [13-15]. Although the number of examined cases was limited, the rate of mucosal healing increased up to 60.0% (57/95) after treatment with CT-P13, while only 29.5% of patients (28/95) were assessed as normal or mild in the initial endoscopic examination.
In this analysis of the PMS of CT-P13, we classified patients into 3 groups (naïve, nonmedical switch, and medical switch) according to previous biologic treatment and reasons for switching, and we evaluated ADRs and efficacy in each group. It could be assumed that each of the 3 groups correspond to the stages of induction, maintenance, and relapse, respectively, and we were able to estimate the usefulness of CT-P13 in each clinical situation.
Firstly, biologic-naïve patients, who had not been treated with anti-TNF-α antibody, responded well to CT-P13. The efficacy parameters decreased promptly after the initiation of administration, and the proportion of responders after induction therapy was high. The low clinical severity of disease in the naïve patients in this PMS made it difficult to compare with the efficacy of the originator IFX in the reported PMS, which included patients with more severe disease. However, recent clinical studies suggest that CT-P13 and the originator IFX have similar efficacy in naïve patients with IBD [16-19].
Secondly, patients who were switched for nonmedical reasons, such as economic reasons or institutional policies, showed very high treatment continuation rates. In fact, only 12 and 5 patients were discontinued from CT-P13 therapy due to ADRs and loss of response, respectively. With previous IFX treatment, the decreased CDAI and partial Mayo score, as well as CRP, were well maintained and even tended to decrease further with CT-P13 treatment. These favorable results are consistent with the previously reported results from European and Korean studies in patients switched for nonmedical reasons strongly suggesting interchangeability of CT-P13 with originator IFX in these patients [20-22].
The last patient group consisted of patients who received CT-P13 after the originator IFX or ADA for medical reasons. The incidence of ADRs and IR in this group was relatively high, especially in patients switched due to AEs. Therefore, therapeutic options other than biosimilar should be considered for the patients with ADRs including IR due to IFX or ADA. Although the proportion of nonresponders in this patient group was high, responders and consistent responders accounted for more than 70% of these patients. The unexpected efficacy of CT-P13 in patients who became less responsive to the originator IFX is unlikely to be due to the differences between the 2 biologics, because pharmaceutical quality control testing and clinical trials of CT-P13 showed very similar results to the originator IFX [3-5]. It is possible that transient fluctuation of disease activity accounts for either loss of response to IFX or improvement by CT-P13. It is reported that reduced response to IFX is mainly explained by decreased trough drug level, induction of anti-drug antibodies, and increased TNF-α at the lesion [23-26]. None of these analytical parameters were monitored in this PMS in standard clinical practice, as is an additional limitation of the PMS. However, taking these factors into account together with the non-severe disease status of the patients in this PMS, transient changes in disease condition may have contributed to the efficacy of CT-P13 in patients with a reduced response to the originator IFX.
In conclusion, this interim analysis of the PMS with 523 patients showed that the incidence and type of ADRs to CT-P13 were similar to those of the originator IFX. It was also shown that CT-P13 was effective in biologic-naïve patients and maintained efficacy after switching from the originator IFX for nonmedical reasons. In patients switched from the original biologics due to AE or insufficient efficacy, the incidence of ADRs and proportion of nonresponders were higher than in other patient groups, but the treatment continuation rate was not significantly different from that in naïve patients. These results showed that CT-P13 is a useful, cost-efficient biosimilar that is comparable to and interchangeable with the originator IFX in patients with IBD.
Notes
FINANCIAL SUPPORT
The authors received no financial support for the research, authorship, and/or publication of this article.
CONFLICT OF INTEREST
Nakagawa T and Suzuki Y have received research support from Nippon Kayaku, Mitsubishi Tanabe, and AbbVie. Kobayashi T has received research support and honoraria from Nippon Kayaku, and honoraria from Mitsubishi Tanabe, AbbVie, and Celltrion. Watanabe M has received research support and honoraria from Nippon Kayaku, Mitsubishi Tanabe, and AbbVie, and honoraria from Celltrion. Hibi T has received research support from Nippon Kayaku, and honoraria from Mitsubishi Tanabe, and AbbVie. Nishikawa K and Yamada F are employees of Nippon Kayaku. Asai S and Sameshima Y have no conflicts of interest to disclose.
AUTHOR CONTRIBUTION
Conceptualization: Suzuki Y, Watanabe M, Hibi T. Formal analysis: Nishikawa K, Yamada F. Investigation: Nakagawa T, Kobayashi T, Asai S, Sameshim Y. Methodology: Suzuki Y, Watanabe M, Hibi T. Validation: Yamada F. Supervision: Hibi T. Visualization: Nishikawa K, Yamada F. Writing - original draft: Nishikawa K. Writing - review and editing: Nakagawa T, Kobayashi T. Approval of final manuscript: all authors.
Acknowledgements
The authors would like to express our sincere gratitude to all the patients and physicians who participated in the Japanese Post-Marketing Surveillance of Infliximab Biosimilar 1 ‘NK’ (CT-P13).