Familial aggregation of inflammatory bowel disease in India: prevalence, risks and impact on disease behavior
Article information
Abstract
Background/Aims
Information about familial aggregation of inflammatory bowel disease (IBD) in Asia is limited. We aimed to analyze the prevalence and risk of familial IBD in an Indian cohort and compare familial and sporadic cases.
Methods
Familial IBD cases were identified from a large prospectively maintained IBD registry. The prevalence of IBD in first- and seconddegree relatives of index cases was evaluated. The disease behavior was compared to that of sporadic cases.
Results
Total 3,553 patients (ulcerative colitis [UC], 2,053; Crohn’s disease [CD], 1,500) were included. Familial IBD was noted in 4.13% of CD and 4.34% of UC patients. Family history was commoner in pediatric group (< 18 years) (P= 0.0002; odds ratio [OR], 2.8; 95% confidence interval [CI], 1.6–4.8). Majority had paternal transmission (UC, 67.42%; CD, 70.97%). Concordance of disease type was higher in UC (79.7%) compared to CD (37.1%). Familial IBD was associated with higher cumulative relapse rate (CD, P< 0.001; UC, P< 0.001), higher cumulative rate of surgery (CD, P< 0.001; UC, P< 0.001) and higher rate of biologic use (CD, P= 0.010; UC, P= 0.015). Pan-colitis was higher in familial UC (P= 0.003; OR, 1.935; 95% CI, 1.248–3.000). Fistulizing disease was commoner in familial CD (P= 0.041; OR, 2.044; 95% CI, 1.030–4.056).
Conclusions
The prevalence of familial IBD in India appears comparable to rest of Asia but lower than the West. It is associated with a younger age of onset, higher incidence of pan-colitis in UC and fistulizing complications in CD. Familial IBD has higher cumulative relapse, surgery and biologic use rates. Hence, family history of IBD could have important prognostic implications.
INTRODUCTION
Ulcerative colitis (UC) and CD are chronic relapsing IBD predominantly of the Western world with an increasing prevalence in the emerging economies of the Asia-Pacific region over the last two decades [1].
The risk of development of IBD is highest among first-degree relatives of patients suffering from IBD [2-5]. In fact a positive family history has been the strongest risk factor for development of IBD with a 10–15 times higher risk in unaffected first-degree relatives [6-8]. Familial clustering of IBD has been related to a composite of shared environmental exposures and genetic influences as proven in twin studies [9-13]. On the other hand, the increasing incidence of IBD in Asia [14,15] has been attributed to Westernization and changing lifestyles suggesting environmental influences as possible causative factors. This has been highlighted by numerous immigration studies from the West and South East Asia [16-20].
Asian IBD has shown genotypic and phenotypic differences from that of the West. The prevalence of familial IBD has ranged between 10% and 25% in Northern European and American populations [21]. On the other hand majority of studies from Asia including Korea, China and Japan have reported a prevalence of 1% to 6% [22-27]. It is not yet clearly understood whether familial IBD is phenotypically different from sporadic IBD in terms of disease behavior and outcomes although a more severe and early onset disease has been suggested [27].
IBD is an emerging disease in India. The known genetic mutations like NOD2 (nucleotide-binding oligomerization domain-containing protein 2) do not appear to be playing a role among the Indian population [28]. Although some genetic risk factors have been explored [29-31], an investigation into the familial relationships and its potential impact on the nature of disease and prognosis has not been examined. Knowledge of disease phenotype of familial IBD may help to identify key genetic, environmental and behavioral factors contributing to the development of IBD in this population.
We aimed to assess the prevalence and the time trends of occurrence of familial IBD in India, the concordance of CD and UC amongst affected family members and whether the phenotype and behavior of familial IBD was distinct from that of sporadic IBD.
METHODS
1. Study Population
The study was conducted at an independent single specialty gastroenterology high volume referral center for IBD patients across India. The IBD Clinic here has a well-established registry of more than 4,000 patients. At the time of analysis, the database included 4,150 patients. We included only those patients who have complete data on family history. All adults and children who are seen at IBD Clinic are invited to participate in the registry with a greater than 99% acceptance rate and documentation of informed consent for research purposes. Patients with incomplete information available on family history of IBD and who did not consent for the study were excluded.
At inclusion, the demographic and disease details are collected by interview and chart review and entered in the database including presenting symptoms, disease extent and activity scores, comorbid conditions, medications and response to treatment. The data is updated at each subsequent visit. The presence of family history of IBD and the degree of relationship are also documented. CD and UC patients with information available on their family history of IBD were included for analysis. The course of the disease was assessed including the number of relapses, need for steroids or biologics and the requirement of surgery.
Relapse in UC was defined as increase in stool frequency and recurrence of rectal bleeding which was confirmed by endoscopy [32]. Relapse in CD was defined as flare of symptoms in a patient with established CD confirmed by laboratory investigations, imaging and endoscopy [33].
All participants received at least one endoscopic procedure at our institute to confirm diagnosis. Disease activity was recorded at each visit using the CDAI for CD and Mayo score for UC. Disease extent was recorded consistent with the Montreal classification. The maximum extent and severity noted at any visit was included for analysis.
Familial IBD was categorized as having a first- or second-degree relative with CD or UC. First-degree relative family history included at least 1 parent, sibling or child with IBD. The number of affected relatives, relationship was collected based on interview and the type, location and activity of the relative’s IBD were retrieved from database or review of treatment records whenever available. Patients with incomplete information on family history of IBD were excluded as mentioned earlier. The family details were validated by confirmation by a family member and available medical records of the relation as available. Patients without affected family member with IBD were considered sporadic IBD.
2. Statistical Analyses
The demographic differences between familial and sporadic IBD were compared using frequencies for categorical variables and median and range for continuous variables. Categorical variables (e.g., familial and sporadic group, UC and CD) were compared between groups using the chi-square test and OR was calculated adjusting for confounding factors. Cumulative relapse rate, surgery rate and biologic use were compared by survival analysis using log-rank test. Statistical analysis was performed using SPSS 21.0 version (IBM Corp., Armonk, NY, USA). All P<0.05 were considered statistically significant.
3. Details of Ethical Approval
This study was approved by the Institutional Review Board of the Asian Institute of Gastroenterology (Ethics committee Registration No: ECR/346/Inst/AP/2013), and informed consent was obtained from all participants. All clinical data and samples were obtained with written informed consent under Institutional Review Board and ethics committee.
RESULTS
1. Patient Demographics
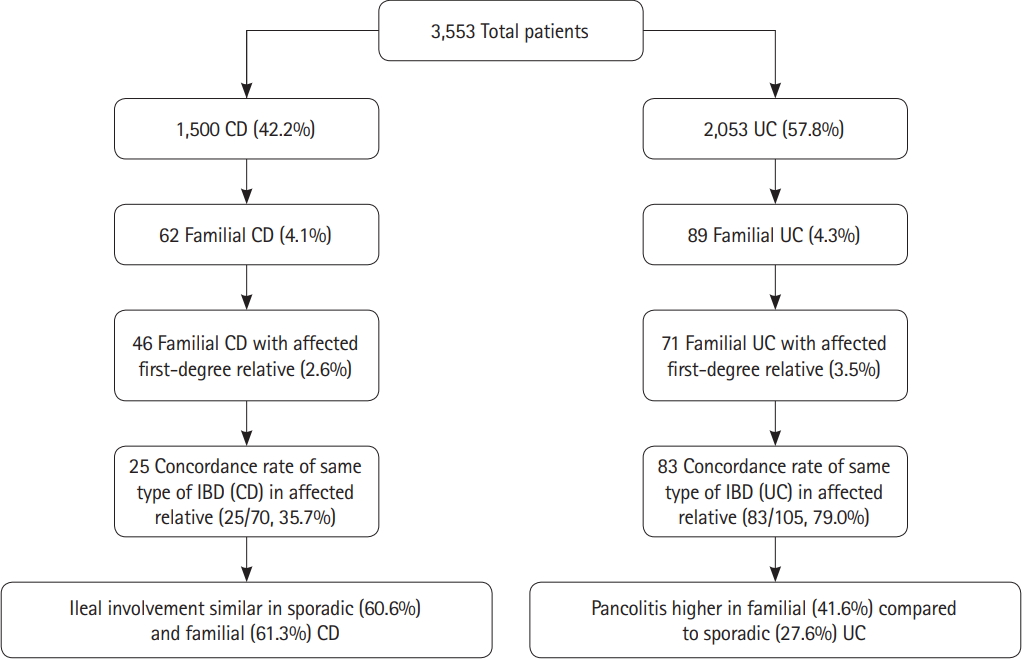
A total of 3,553 patients were included in the study (CD, 1,500; UC, 2,053). UC was more prevalent affecting 57.78% of the cohort population. Majority of patients were males 2,340 (65.86%) (Table 1, Fig. 1).
2. Prevalence of Familial IBD
Overall 151 out of 3,553 patients (4.25%) had familial IBD (4.13% of CD and 4.34% of UC patients). The remaining 3,402 patients were considered to be sporadic IBD (Table 1). There was no difference in the age of onset of symptoms, age at clinical diagnosis or disease duration between sporadic and familial IBD.
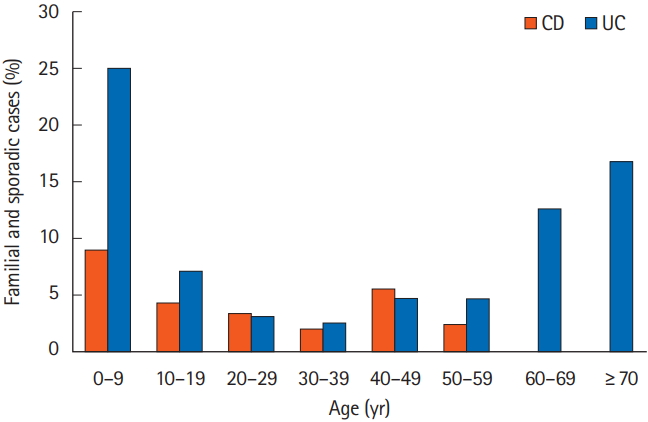
The prevalence of family history according to the age of onset is shown in Fig. 2. The maximum number of cases for both CD and UC were in the 0–9 years age group. A total of 223 patients were in the pediatric age group (age of onset <18 years). Sixteen of the pediatric age group (7.17%) had a positive family history compared to 135 of 3,330 (4.05%) of the adult patients (age of onset ≥18 years). This was significantly higher in the pediatric age group (P=0.002; OR, 2.8; 95% CI, 1.6–4.8). Majority of affected family members were first-degree relatives (3.27% of CD and 3.51% of UC patients) (Table 2).
3. Distribution of Relationships
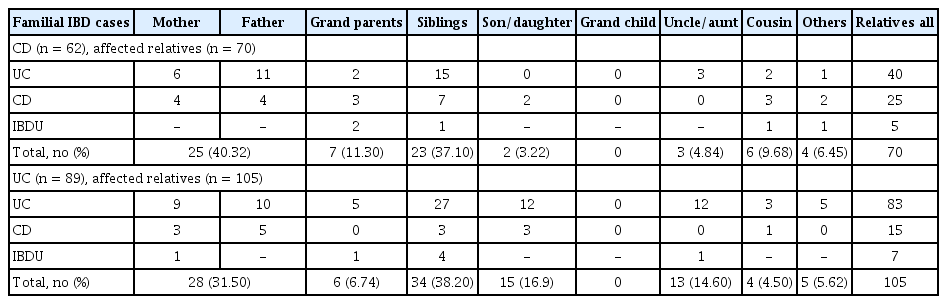
The distributions of relationships are shown in Table 3. For CD, parents and sibling was most prevalent at 40.32% each followed by grandparents at 11.3% and smaller numbers by other groups. Similarly, for UC the highest percentage was noted in first-degree relatives; parents 31.5%, sibling 38.2% and child 16.9%. A person’s parent, sibling, or children were considered as the first-degree relatives. The paternal side was more frequently involved for both UC and CD. The paternal side was affected in 70.97% for patients with CD and 67.42% in UC patients. While considering only first-degree relatives, the paternal side was affected in 78.26% of patients with CD and 81.7% of patients with UC (Table 4).
4. Concordance of Disease Types between Probands and Affected Relatives
Sixty-two probands with CD had 70 relatives with IBD (25 CD, 40 UC, and 5 IBD unclassified). Concordance of CD in patients with any affected blood relatives was 37.1% for CD. Similarly, 89 probands with UC had 105 relatives with IBD (83 UC, 15 CD, and 7 IBD unclassified). The concordance was 79.7% for UC (Table 2). Among patients with family history of IBD in first-degree relatives, concordance for CD was noted in 34.7%. For UC the disease concordance was 76.05% of first-degree relatives (Table 4).
5. Location and Disease Behavior of Sporadic versus Familial IBD
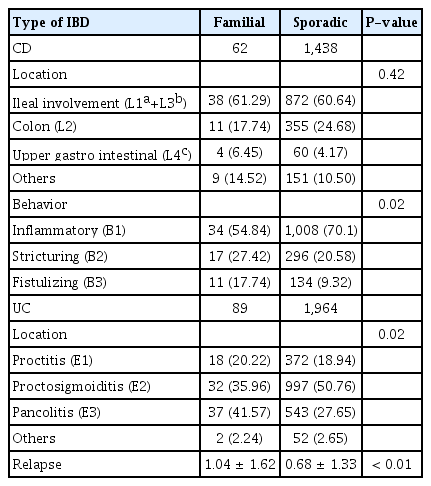
Pan-colitis was more common in 37 out of 62 (41.57%) of familial UC compared to 543 out of 1,438 (27.65%) in sporadic cases (P=0.003; adjusted OR, 1.935; 95% CI, 1.248–3.000) (after adjusting for age, sex and duration of disease). Proctosigmoiditis (E2) was also more common in familial UC whereas proctitis (E1) was similar in between the 2 groups. Ileal involvement was similar in 38 out of 62 patients with familial CD (61.29%) compared to 872 out of 1,438 patients (60.64%) with sporadic CD (adjusted OR, 0.936; 95% CI, 0.552–1.587; P=0.806). There was no difference in inflammatory, stricturing disease between familial and sporadic CD.
Fistulizing CD was more common in familial CD compared to sporadic CD (P=0.041; adjusted OR, 2.044; 95% CI, 1.030–4.056) (Table 5).
6. Comparison of Disease Activity
Comparison of disease activity based on partial Mayo score at index visit between the familial UC (mean, 5.36; SD, 2.58) and sporadic CD (mean, 5.24; SD, 2.44) did not show significant difference (P=0.44). Similarly, comparison of disease activity based on CDAI score at index visit between the familial CD (mean, 209.89; SD, 80.419) and sporadic CD (mean, 227.57; SD, 79) did not show significant difference (P=0.08).
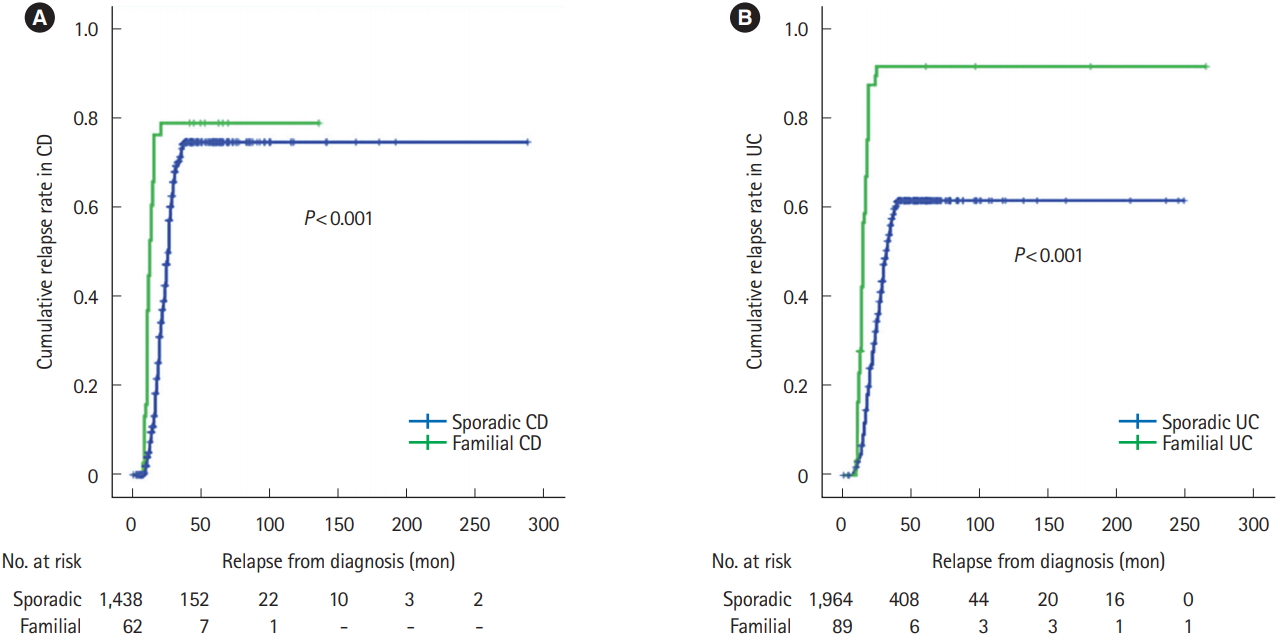
7. Cumulative Relapse Rate, Cumulative Rate of Surgery and Biologics Use
Familial IBD was associated with higher cumulative relapse rate than sporadic IBD in both CD (P<0.001) and UC (P< 0.001) (Fig. 3). Cumulative rate of surgery was higher in familial CD compared to sporadic CD (P<0.001) and familial UC compared to sporadic UC (P<0.001) (Fig. 4). Use of biologics were also higher in familial IBD compared to sporadic IBD with time (CD, P=0.010; UC, P=0.015) (Fig. 5). Most patients received infliximab or an adalimumab biosimilar.
DISCUSSION
It is now well accepted that the greatest single risk factor for development of IBD in any individual is having an affected family member [9]. The extent of this risk and prevalence has varied between different Asian countries and from the Western world likely due to a varying genetic predisposition and environmental factors. It is not clearly understood whether familial IBD is a distinct entity from sporadic cases although phenotypic differences in disease behavior and severity between familial and sporadic IBD has been suggested.
There is very limited data on familial aggregation of IBD from India and its impact on disease behavior has not been evaluated. Our study is the first large scale study from India on the familial aggregation of IBD and on the differences in disease behavior between sporadic and familial IBD. We analyzed a well-established and prospectively maintained database of 3,553 Indian patients with IBD and identified 151 patients (4.25%) with a positive family history of IBD including 4.13% for CD and 4.34% for UC. An earlier Indian Society of Gastroenterology Task Force questionnaire-based survey of IBD in general found a prevalence of 2.9% [34]. These prevalence rates are comparable with most studies from Asia including Japan (2.7% of UC and 2.6% of CD) [23], China (1.48% of UC) [25], and Hong Kong (3% of overall) [14]. A recent study from Korea suggested higher prevalence rates of 6.8% for CD and 6.5% for UC [15] which was still lower than United States and the Northern Europe [27].
Overall the prevalence in Asia appears significantly lower than those reported from the West with values ranging from 8% to 13.8% for UC and 5% to 15% for CD.4, 9, 11,13,21 Wang et al. [26] reported a striking difference in incidence of family history of IBD between China (4.1%) and United States (39.3%). A meta-analysis involving 71 studies with 86,824 patients (mostly from Western countries) showed a prevalence of around 12% in UC [35].
Additionally, most Western studies have suggested a higher prevalence of familial IBD in CD compared to UC [6,7,9,10] which this was not noted in the Asian context. In our study the family history was marginally higher in UC patients than CD. Kuwahara et al. [23] from Japan, reported similar results (2.7% of UC and 2.6% of CD).
We found the risk of IBD to be the highest among first-degree relatives, as would be expected with a genetic predisposition or an early life environmental exposure shared by family members. Parents and siblings were the most affected relatives (37.8% each) followed by grandmother, cousin, child and uncle or aunt in decreasing order. A number of other studies have reported a higher prevalence among first-degree relatives compared with second-degree or distant relatives [36-38]. However, a recent meta-analysis showed that prevalence was independent of first-degree, second-degree or distant relationship [39].
Interestingly, majority of patients showed a paternal predisposition and most of the affected relatives were from paternal rather than maternal side both in UC and CD. This was quite different from other observations where there was either high maternal transmission or equal on both sides [39-41]. Genetic anticipation like trinucleotide repeat disorders can occur in CD evidenced by paternal transmission, younger age and a greater extent of disease at onset in the younger member of two-generation pairs affected with CD [42]. This could be a possible reason for the high paternal transmission in our cohort as well.
The concordance of disease type or the tendency of the patient of having the similar type of IBD as the affected relative was significantly higher for UC (78.3%) than with CD (40.5%) in our study. A high concordance rate for both subtypes of IBD has been suggested in several studies. Satsangi et al. [40] reported concordance for type of disease in 75.30% of affected parent-child pairs, Roma et al. [43] showed 68% concordance in 22 pairs of first-degree relatives for the disease phenotype. A recent study from Northern India based on telephonic survey however reported a 100% concordance rate in UC among family members [44]. Phenotypic differences of IBD between the north and south of the India could be a plausible reason but there is no conclusive decision on the same [45].
Overall familial IBD patients had an earlier age of onset than sporadic IBD. The prevalence was highest in 0–9 years age group. These findings corroborate with earlier studies primarily from Japan [23,46]. Here again genetic anticipation whereby the symptoms of a genetic disorder manifests at an earlier age with each generation could play a role [42,47,48]. Clinically this could predict an aggressive disease course in familial cases as young age is a known predictor of severe disease.
The phenotypic differences between familial and sporadic IBD is difficult to interpret primarily because of the wide ranges in estimates across studies [36]. Many Asian countries have reported a more severe disease course of familial IBD compared to sporadic cases. Familial CD in Korea had higher anti-TNF use and early intestinal resection compared to sporadic CD [22]. Similarly, Kuwahara et al. [23] found familial cases had an aggressive disease course compared to sporadic (both UC and CD) in Japan. This is in accordance to our finding as we have found that familial IBD has aggressive course compared to sporadic IBD as evidenced by higher cumulative relapse rate, higher rate of intestinal surgery and early use of biologics.
This was in contradiction to earlier studies by Henriksen et al. [49] who found that family history did not influence disease phenotype and was not a prognostic factor of disease activity. In a similar study from the West in CD it was shown that familial CD was not different from sporadic CD in terms of disease behavior if non-stricturing and non-fistulizing cases are excluded [50].
We also analyzed the influence of family history on disease behavior. The incidence of fistulizing disease was significantly higher in familial CD with higher number of relapses. In familial UC, the incidence of pan-colitis, number of relapses and steroid usage were significantly higher compared to sporadic UC.
Interestingly there was no significant difference in the use of biologics between the familial and sporadic UC in our study though the use of steroids and number of relapses were significantly higher. The restricted usage of biologics due to risk of tuberculosis and other infectious complications could play a role. Additionally, the use of biologics in India is often governed by the cost since majority of patients pay out of their pockets. Affordability rather than a milder disease results in lower usage of expensive biologics.
Our study does have some limitations. Firstly, this was not a population-based study limiting our ability to generalize our findings to the general population though this was a large cohort of patients from across the country. There is a definite possibility of overestimation of familial cases since it was based out of a large tertiary care referral center with potentially more severe cases. We however did not find major differences from other Asian studies including an earlier questionnaire-based survey from India which had a single yes/no question option for family history. We could not calculate the lifetime risk of IBD in the population because there is no population-based registry in the country. The only available incidence data from the country is a survey done for UC in North India in 2002 [51]. Additionally there is always a probability of recall bias. We actually considered familial IBD only up to second-degree relatives to minimize this risk. The family history of UC or CD was obtained from the patient himself as reported. We had no means to verify the diagnosis of CD or UC in all family members.
IBD is on the rise in the developing countries including India. This is the first study analyzing familial IBD in India to assess phenotypical and behavioral differences with sporadic IBD. It shows that familial IBD in India is similar to that of the other Asian countries suggesting similar genetic and environmental influences in this part of the world but clearly distinct from that reported from the West.
Familial IBD was associated with an earlier onset of disease with increased severity of disease. This was particularly significant in UC with higher number of relapses and need for steroids. These findings have important implications for clinical practice and would help the clinician to predict the course of disease, stratify familial IBD as high risk and initiate treatment accordingly.
Notes
FINANCIAL SUPPORT
Hutfless S received support from the Ludwig-Bayless award.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION
Conceptualization, methodology: Banerjee R. Formal analysis: Pal P. Funding acquisition, project administration, supervision: Banerjee R, Reddy DN. Visualization: Banerjee R, Ganesh BG. Data curation: Ganesh BG. Writing - original draft: Banerjee R, Pal P, Hutfless S. Writing - review and editing: Banerjee R, Pal P, Hutfless S, Reddy DN. Approval of final manuscript: all authors.