Intestinal Permeability Regulation by Tight Junction: Implication on Inflammatory Bowel Diseases
Article information
Abstract
Epithelial tight junctions (TJs) are the key structures regulating paracellular trafficking of macromolecules. The TJ is multi-protein complex that forms a selective permeable seal between adjacent epithelial cells and demarcates the boundary between apical and basolateral membrane domains. Disruption of the intestinal TJ barrier, followed by permeation of luminal noxious molecules, induces a perturbation of the mucosal immune system and inflammation, which can act as a trigger for the development of intestinal and systemic diseases. Inflammatory bowel disease (IBD) patients demonstrate increased intestinal paracellular permeability. Although it remains unclear whether barrier dysfunction precedes disease or results from active inflammation, increased intestinal TJ disruption is observed in IBD patients suggest that dysregulation of TJ barrier integrity may predispose or enhance IBD progression. Therefore, therapeutic target to restore the TJ barrier integrity may provide effective therapeutic and preventive approaches against IBD. This review discusses the molecular structure and regulation of intestinal TJs and the involvement of intestinal TJs in IBD pathogenesis.
INTRODUCTION
Intestinal permeability is the property that allows solute and fluid exchange between the lumen and tissues. Conversely, intestinal barrier function refers to the ability of the mucosa and extracellular barrier components, such as mucus, to prevent this exchange.
A critical function of the intestinal epithelium is to form a barrier that prevents permeation of pro-inflammatory molecules, such as pathogens, toxins, and antigens, from the luminal environment into the mucosal tissues and circulatory system.1 Barrier defects have been associated with a variety of human diseases, including those primarily affecting the gut, such as IBD, celiac disease, and IBS, as well as systemic diseases or diseases involving other organ systems, such as type I diabetes, acquired immunodeficiency syndrome (AIDS), multiple sclerosis, and rheumatoid arthritis.2,3,4,5,6,7
Epithelial tight junctions (TJs) maintain the intestinal barrier while regulating permeability of ions, nutrients, and water. The TJ is a multi-protein complex that forms a selectively permeable seal between adjacent epithelial cells and demarcates the boundary between apical and basolateral membrane domains.8 The modification of TJ barrier function and paracellular permeability is dynamically regulated by various extracellular stimuli and is closely associated with our healthy and susceptibility disease.1,9 TJ barrier disruption and increased paracellular permeability, followed by permeation of luminal pro-inflammatory molecules, can induce activation of the mucosal immune system, resulting in sustained inflammation and tissue damage.
Evidence from basic science and clinical studies indicate that the intestinal TJ barrier has a critical role in the pathogenesis of intestinal and systemic diseases.1,10 Thus, this review summarizes the molecular structure and regulation of intestinal TJs and the involvement of intestinal TJs in IBD pathogenesis.
STRUCTURES AND FUNCTIONS OF THE TJ PROTEINS
TJs are multiple protein complexes located at the apical ends of the lateral membranes of intestinal epithelial cells (Fig. 1).11,12 Four integral transmembrane proteins, occludin,13 claudins,14 junctional adhesion molecule (JAM),15 and tricelluin,16 have been identified. The intracellular domains of these transmemebrane proteins interact with cytosolic scaffold proteins, such as zonula occludens (ZO) proteins, which in turn anchor the transmembrane proteins to the actin cytoskeleton. The interaction of TJ proteins with the actin cytoskeleton is vital to the maintenance of TJ structure and permits the cytoskeletal regulation of TJ barrier integrity.17 The circumferential contraction and tension in the actin is regulated by myosin light chain (MLC) activation.18 Induction of MLC phosphorylation by kinases such as MLC kinase (MLCK) and Rho-associated kinase causes the contraction of the actin, resulting in the opening of the paracellular pathways.18,19
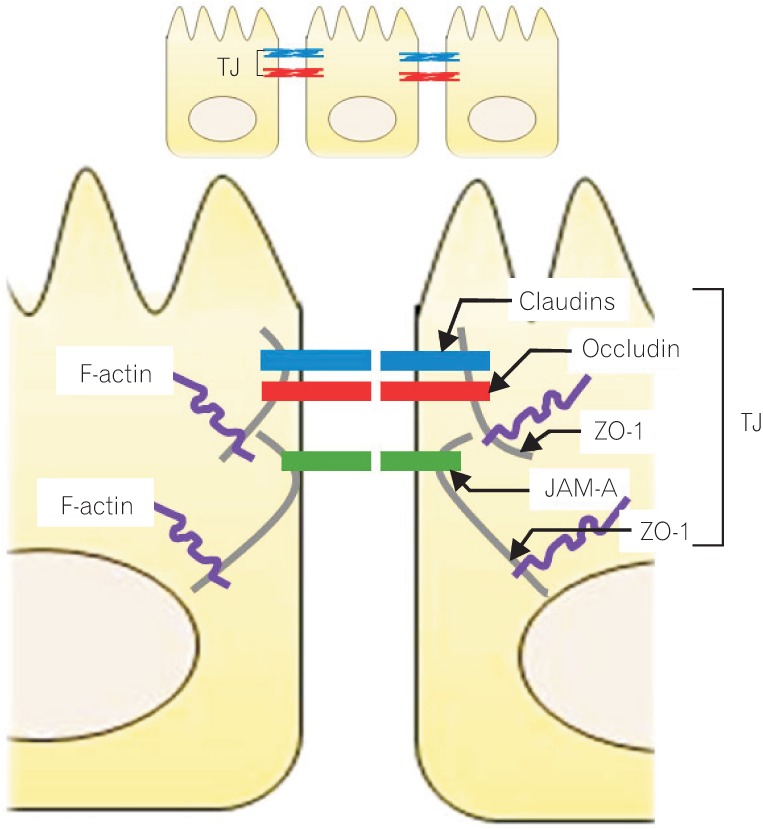
Molecular structure of the intracellular junction of intestinal epithelial cells. The tight junctions (TJs), multiple protein complexes, locate at the apical ends of the lateral membranes of intestinal epithelial cells. The TJ complex consists of transmembrane and intracellular scaffold proteins. The transmembrane proteins (claudins, occludin, and junctional adhesion molecules [JAMs]) create a permselective barrier in the paracellular pathways. The intracellular domains of the transmembrane proteins interact with the intracellular scaffold proteins such as zonula occludens (ZO) proteins, which in turn anchor the transmembrane proteins to the actin cytoskeleton.
1. Occludin
Occludin was the first integral membrane TJ protein.13 The long C-terminal domain of occludin interacts with several intracellular TJ proteins, such as ZO proteins, which are required to link occludin to the actin cytoskeleton (Fig. 2).20 The function of occludin is not yet fully understood, but numerous studies using animals and cell cultures indicate that it has crucial roles in the TJ structure and permeability in the intestinal epithelia.21
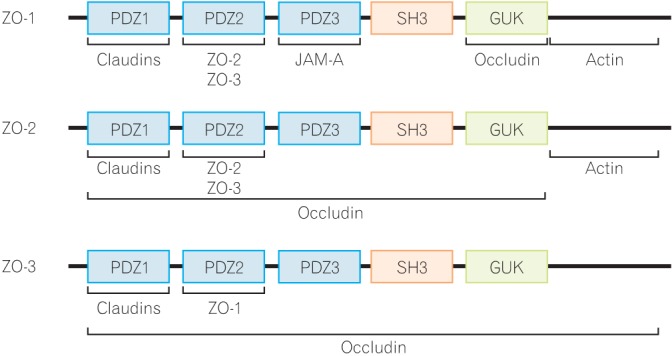
Interaction of proteins with the integral scaffold tight junction (TJ) proteins, zonula occludens (ZO)-1, -2, and -3. ZO proteins carry 3 post-synaptic density 95/Drosophila disc large/zona-occludens (PDZ) domains, a Src homology-3 (SH3) domain, and a region of homology to guanylate kinase (GUK) from the side of the N-terminus. Several TJ proteins and cytoskeletal actin interact with the ZO proteins. JAM-A, junctional adhesion molecule-A.
Recent studies showed that knockdown of occludin induces an increase in paracellular permeability to macromolecules, which indicates that occludin plays a role in the maintenance and assembly of TJs.21 In the intact epithelium, occludin is highly phosphorylated on the serine and threonine residues,22 and the phosphorylation has a role in the maintenance and assembly of the TJ structure.23 Some kinases, such as protein kinase C (PKC) and casein kinase, have been identified as responsible for its phosphorylation.24,25 In addition, although the level of tyrosine-phosphorylated occludin is very low in the intact epithelium, several studies have shown that tyrosine phosphorylation is caused during disassembly by various stimuli.26
Underlying this mechanism, the tyrosine phosphorylation of occludin attenuates the interaction with ZO-1, leading to dissociation from the junctional complex. Like hydrogen peroxide, acetaldehyde, a biological metabolite of ethanol, also induces TJ disruption in intestinal epithelial cells in an occludin tyrosine phosphorylation-dependent manner.27
2. Claudins
Claudins do not have any sequence similarity to occludin. The extracellular loops of claudin molecules make hemophilic and heterophilic interactions with adjacent cells and the interactions create either barriers against or pores for the passage of selective molecules in the paracellular pathways.12,28 Numerous studies have demonstrated that claudins are the key component and backbone of TJs. When claudins are expressed in fibroblast, they are incorporated into TJ strands and form paired strands at the cell-cell contacts.14
Recent studies using claudin knockout mice have shown that claudins play a critical role in barrier formation and paracellular permeable selectivity in various tissues.29 As a prominent example, claudin-1 knockout mice die within 24 hours of birth because of a dramatic loss of fluid and electrolytes through the impaired epidermal barrier. Claudins are a multigene family with at least 24 members in humans and mice, and each isoform shows a unique expression pattern in tissues and cell lines. As is the case with occludin, some claudin isoforms are phosphorylated in the cells,30 and this phosphorylation is associated with localization and paracellular permeability.
3. Junctional Adhesion Molecule
The JAM family belongs to the Ig superfamily and is characterized by 2 extracellular Ig domains, one transmembrane domain, and one intracellular C-terminal domain.31 The extracellular N-terminal domains of the JAM family members bind to various ligands through hemophilic and heterophilic interactions.32 The hemophilic interactions involving the JAM members have a role in the formation of TJs and the cell-cell border. In contrast, the heterophilic interactions function in cell-cell adhesion, association between leukocytes and epithelial/endothelial cells, platelet activation, and virus recognition.
JAM members are expressed in various cell types including epithelial, endothelial, and immune cells, and exhibit different expression patterns in both a tissue- and cell type-specific manner. In intestinal epithelial cells, JAM-A and JAM-4 are expressed and involved in TJ regulation. In vitro and in vivo studies demonstrate that JAM-A participates in the regulation and maintenance of the TJ barrier. Treatment of intestinal epithelial cells with monoclonal JAM-A antibodies inhibited the resealing of the TJs, as indicated by delays in transepithelial electrical resistance (TER) recovery and occludin assembly.33 Recent studies have shown that JAM-A knockout mice exhibit higher permeability to dextran and myeloperoxidase activity in the colon compared to wild type mice. In addition, the colonic injury and inflammation induced by dextran sodium sulfate are more severe in the JAM-A knockout mice than in wild type mice.34
4. Zonula Occludens
The ZO proteins were the first TJ-specific proteins identified and three ZO proteins, ZO-1, -2, and -3, have been identified to date.35 According to sequence analysis, these ZO proteins are categorized as members of the membrane-associated guanylate kinase homolog family.17 They are multi-domain proteins carrying three post-synaptic density 95/Drosophila disc large/zona-occludens (PDZ) domains, a Src homology-3 domain and a region of homology to guanylate kinase homolog from the side of the N-terminus (Fig. 2).36 These multi-domain structures provide an intracellular scaffold in the TJs and are required for regulation and maintenance of TJ structure.
Interestingly, many TJ proteins bind to the N-terminal half region of ZO proteins, while the C-terminal region interacts with the actin cytoskeleton and cytoskeleton-associated proteins.37 Among the ZO proteins, the biochemical function and property of ZO-1 have been well examined. ZO-1 localizes to the nascent cell-cell contacts in both cell cultures and animal models. Therefore, it has been proposed that ZO proteins may mediate the early assembly of TJ proteins into cell-cell contacts. To data, intensive efforts have been made to clarify the functional role of ZO proteins, but it has been difficult to obtain clear evidence showing the importance of ZO proteins in TJ regulation. Recent studies have been shown that ZO-1 deficient cells are still able to form normal TJ structures and show normal permeability; however, an obvious delay in the assembly of other TJ proteins including occludin and claudins into the TJ is observed, indicating that the ZO proteins have an important role in the regulation of TJ assembly.38
REGULATION OF THE TJ PROTEINS
The roles of cytokines in intestinal TJ regulation under pathophysiological conditions have been well investigated. The cytokine-mediated dysfunction of the TJ barrier, resulting in immune activation and tissue inflammation, is thought to be important in the initiation and/or development of several intestinal and systemic diseases.1,10 In contrast, some growth factors play roles in protection and maintenance of TJ integrity.
1. Interferon-γ
Interferon-γ (IFN-γ) is mainly involved in the regulation of inflammatory immune responses, and levels are elevated in the intestinal mucosa in patients with IBD.39 Recent studies have also demonstrated that IFN-γ increases paracellular permeability in intestinal epithelial cells through the redistribution and expression of TJ proteins and the rearrangement of the actin cytoskeleton.40 Thus, IFN-γ increases actinmyosin contractility in a Rho-associated kinase-dependent manner and induces TJ protein internalization, resulting in intestinal TJ disruption.
2. Tumor Necrosis Factor-α
Tumor necrosis factor-α (TNF-α) is a proinflammatory cytokine that is produced mainly by activated T cells and macrophages. Clinical studies show that TNF-α is elevated in the intestinal mucosa, serum, and stools of IBD patients. TNF-α is known to induce apoptosis and inflammatory response in intestinal epithelial cells,41 and recent studies have also demonstrated that it impairs the intestinal TJ barrier through different mechanism. In intestinal Caco-2 cells, MLCK expression, which has an important role in TJ regulation, was interrelated with the TNF-α-induced barrier defect. The TNF-α-induced decrease in TER is well correlated with MLCK expression and MLC phosphorylation.42 In addition, in intestinal HT-29/B6 cells, TNF-α was seen to decrease TER through claudin-2 expression.43 Claudin-2 is expressed throughout the intestine and forms a pore for cations, which is blocked by involvement of phosphatidyl inositol-3 kinase (PI3K)/Akt signaling.
3. Interleukin-1β
Interleukin-1β (IL-1β) is markedly elevated in intestinal mucosa under inflammatory conditions, such as CD patients.44 In clinical studies, an IL-1 receptor antagonist is currently being developed for therapeutic usage, which indicates IL-1β plays a central role in the intestinal inflammatory process.45 Furthermore, recent studies show that IL-1β causes increased intestinal TJ permeability. In intestinal Caco-2 cells, IL-1β decreases TER and increases inulin flux, which is in part mediated by the decreased expression and redistribution of occludin and increased expression of MLCK and MLC phosphorylation.46 It was also shown that the knockdown of nuclear factor-κB p65 inhibits the decrease in occludin and increases in MLCK expression, indicating nuclear factor-κB-dependent transcriptional regulation by IL-1β.47
4. Interleukin-6
The major source of interleukin-6 (IL-6) seen in IBD has been shown to be intestinal epithelial cells and lamina propria mononuclear cells.48 Recent studies indicate the involvement of IL-6 in intestinal TJ regulation.49 IL-6 increases paracellular permeability selectively to cations, but not to macromolecules, with an increase in pore-forming claudin-2 in intestinal epithelial cells, which requires the mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK) and PI3K/Akt signaling activations.
5. Interleukin-10
Interleukin-10 (IL-10) is regarded as an anti-inflammatory cytokine. In vitro studies have demonstrated that IL-10 opposes the cellular functions induced by TNF-α and IFN-γ.50 IL-10 knockout mice, which are widely used as a model of spontaneous colitis, show increased intestinal permeability with elevated TNF-α, IL-1β, and IL-6 expression prior to exhibiting histologic signs of intestinal inflammation.51 These observations suggest that IL-10 has a role in the protection of the intestinal barrier. Clinical and animal studies have demonstrated that a major physiological consequence of total parenteral nutrition is the loss of intestinal barrier integrity.52 Mice administrated with total parenteral nutrition show increased intestinal paracellular permeability and decreased levels of TJ proteins, which is reversed by IL-10 treatment.53 Collectively, IL-10 restores the intestinal barrier defect.
6. Interleukin-17
Interleukin-17 (IL-17) is mainly produced in Th17 cells, potently induces tissue inflammation, and is associated with the pathogenesis a variety of autoimmune conditions, including rheumatoid arthritis, multiple sclerosis, and IBD.54 Information regarding intestinal barrier regulation by IL-17 is limited, but one study has demonstrated that IL-17 induces claudin expression.55 In intestinal T84 cells, IL-17 increases 2 claudin isoforms, claudin-1 and -2, which have opposing effects on TJ integrity. The MEK pathway is involved in IL-17-induced claudin-2 expression, but not claudin-1 expression.
7. Epidermal Growth Factor
Epidermal growth factor (EGF) is initially synthesized as a precursor transmembrane protein, and the mature and soluble form is released by proteolytic cleavage. The EGF receptor is expressed on the membrane surface of most cell types, and EGF mediates various biological functions, including cellular proliferation, differentiation, and survival through the receptor. Further, recent studies have shown that EGF protects intestinal barrier function against noxious stimuli including oxidative stress, ethanol, and acetaldehyde.56 EGF is known to activate several signaling pathways such as PKC and mitogen-activated protein kinase. The activation of the MEK/ERK pathway seems to be required for the EGF-mediated protection of the intestinal barrier against the oxidative stress. Acetaldehyde also disrupts barrier function, which is inhibited by pretreatment of EGF through the activation of EGF receptor-phospholipase-γ-PKCβ1/ε and EGF receptor-MEK/ERK signaling pathways.57
8. Transforming Growth Factor-β
Transforming growth factor-β (TGF-β) is a highly pleiotropic cytokine and proposed to act as a cellular switch to regulate immune function, proliferation, and epithelial mesenchymal transition. Several studies have demonstrated that TGF-β has protective or promotive effects on intestinal barrier function.58 In intestinal T84 cells, TGF-β increases the basal TER, which is mediated by claudin-1 expression through MEK/ERK signaling. In contrast, TGF-β protects the intestinal TJ barrier against noxious stimuli including cryptosporidium parvum and enterohemorrhagic Escherichia coli. In T84 cells, enterohemorrhagic E. coli disrupts the TJ barrier by disturbing the expression and distribution of TJ proteins, including ZO-1, caludin-2, and occludin, which is attenuated by pre-treatment of TGF-β.
INVOLVEMENT OF THE TJ IN IBD
IBD, including UC and CD, are chronic and relapsingremitting inflammatory diseases. Although the exact cause of IBD remains unknown, genetic susceptibility, environmental factors, and immune dysregulation all contribute to disease pathogenesis. In addition, IBD patients demonstrate increased intestinal paracellular permeability, which reflects decreased epithelial barrier function in IBD.59 Clinical studies show decreased expression and redistribution of barrierforming proteins, including occludin, claudin-3, -5, -8, and JAM-A, and increased expression of pore-forming claudin-2 in CD patients.60,61 In UC patients, decreased expression and redistribution of occludin, claudin-1, -4, and JAM-A, and increased caludin-2 expression are observed.61 In addition, MLCK expression and MLC phosphorylation are also observed in the intestines of both UC and CD patients, indicating that cytoskeletal dysregulation is involved in the increased intestinal permeability of IBD patients.62
Clinical studies show that the levels of inflammatory cytokines, including TNF-α,63 IFN-γ,39 IL-1β,44 IL-6,64 and IL-1754 are elevated in the intestines of IBD patients. As described above, these cytokines are known to disturb intestinal barrier function. In particular, TNF-α may have an important role in the initiation and development of intestinal barrier defects in IBD, because anti-TNF-α treatment suppresses the inflammatory response and improves intestinal permeability.65 As a consequence of perturbation of multiple cytokines, the alterations to the intestinal TJ structures in IBD patients could be complicated. In intestinal HT-29/B6 cells, TNF-α treatment resulted in decreased TJ strand number and complexity and increased frequency of strand breaks.66 TNF-α also inhibits occludin promoter activity43 and causes redistribution of occludin, ZO-1, and claudin-1.67 In vitro and in vivo, transcription and translation of MLCK, the major effector responsible for TNF-α-induced TJ modulation, are increased by TNF-α.43,67,68 Other pro-inflammatory cytokines may also mediate barrier function through modulation of MLCK activity. Since represents a common effector used by multiple cytokines to modulate paracellular permeability, it might be an important target for further therapies to restore barrier function during active disease.
CONCLUSIONS
Epithelial barrier dysfunction and inflammation are major contributors to the pathogenesis intestinal disease. Intestinal TJ structures are organized by multiple integral proteins and signaling molecules. Clinical and experimental studies demonstrate that defects in the intestinal TJ barrier and increased permeability are observed in various intestinal and systemic diseases. Intestinal TJ barrier is dynamically regulated by cytokines and pathogens. Therefore, understanding the molecular mechanisms underlying TJ regulation by the extracellular factors will lead to the development of effective therapeutic and preventive approaches against diseases associated with intestinal barrier defects such as IBD.
Notes
Financial support: This study was supported by a grant from Wonkwang University 2013.
Conflict of interest: None.