Risk factors for severity of colonic diverticular hemorrhage
Article information
Abstract
Background/Aims
Colonic diverticular hemorrhage (DH) was a rare disease until the 1990s, and its incidence has increased rapidly since 2000 in Japan. In recent years, colonic DH has been the most frequent cause of lower gastrointestinal bleeding (LGIB). Nearly all cases of DH are mild, with the bleeding often stopping spontaneously. Some cases, however, require surgery or arterial embolization. In this study, using a cohort at Fukuoka University Chikushi Hospital, we investigated factors associated with severe colonic DH.
Methods
Among patients with LGIB who underwent colonoscopy at our hospital between 1995 and 2013, DH was identified in 273 patients. Among them, 62 patients (22.7%) were defined as having severe colonic DH according to recurrence of bleeding in a short period, and/or the necessity of transfusion, arterial embolization, or surgery. We then evaluated risk factors for severe DH among DH patients in this retrospective cohort.
Results
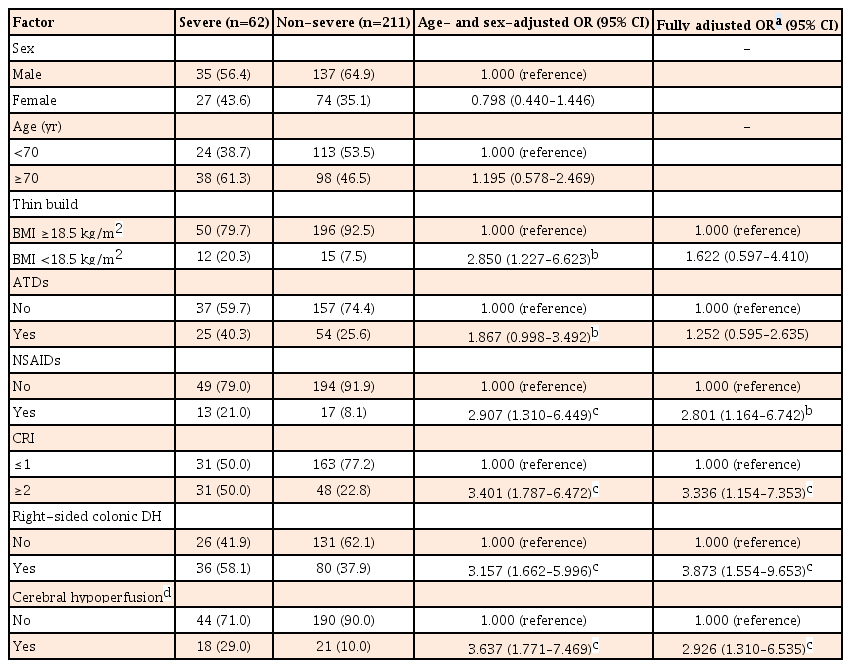
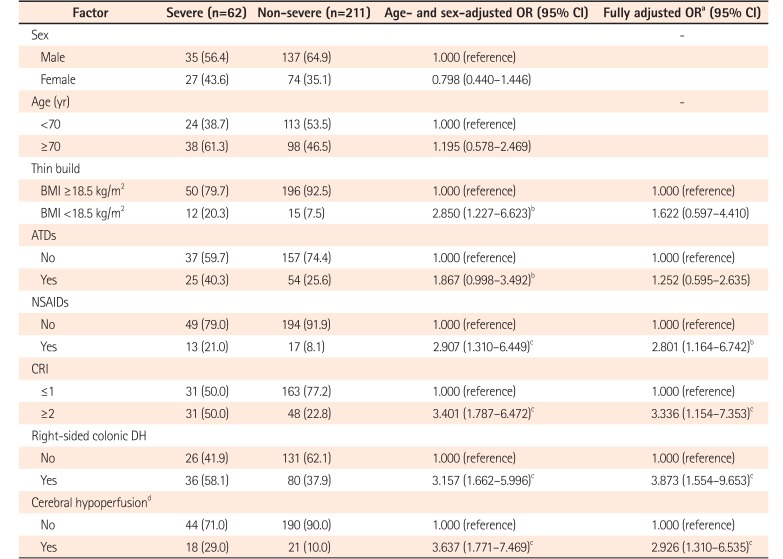
Among the 273 patients with DH, use of non-steroidal anti-inflammatory drugs (NSAIDs) (odds ratio [OR], 2.801; 95% confidence interval [CI], 1.164–6.742), Charlson Risk Index (CRI) ≥2 (OR, 3.336; 95% CI, 1.154–7.353), right-sided colonic DH (OR, 3.873; 95% CI, 1.554–9.653), and symptoms of cerebral hypoperfusion (such as light-headedness, dizziness, or syncope) (OR, 2.926; 95% CI, 1.310–6.535) showed an increased risk of severe DH even after controlling for other factors.
Conclusions
Severe DH occurred in 23% of DH patients, and NSAID use, CRI ≥2, right-sided colonic DH, and symptoms of cerebral hypoperfusion are suggested to be predictors of severe DH.
INTRODUCTION
The use of oral antithrombotic drugs (ATDs) and NSAIDs is increasing with the aging of the population. The number of patients with conditions causing lower gastrointestinal bleeding (LGIB) is also increasing in developed countries, including Japan.1234 In particular, the prevalence of colonic diverticulum is increasing in Japan. Around 75% to 80% of patients with colonic diverticula remain asymptomatic throughout life.5 A small number of these patients develop complications such as diverticulitis or diverticular perforation, but the most common complication is diverticular hemorrhage (DH).5 DH is also increasing in Japan, accounting for 28% to 47% of LGIB cases since 2000, and is now one of the most common causes of LGIB.126
We previously reported risk factors for DH in patients with diverticulosis.1 However, we did not evaluate risk factors for severe DH among these patients. The present study aims to identify factors associated with severity of DH among colonic DH patients.
METHODS
1. Patients and Subjects
Between January 1995 and December 2013, a total of 42,540 patients underwent colonoscopy (CS) at Fukuoka University Chikushi Hospital, one of the main emergency hospitals in Chikushino City, Fukuoka, Japan.1 The present retrospective study included all patients who underwent CS for overt LGIB during this 19-year period. As reported previously,1 273 patients (172 men and 101 women) received a diagnosis of DH (207 confirmed colonic DH and 66 suspected colonic DH). They were 69.0±13.3 years old at diagnosis. In this study, severe DH was defined as the need for blood transfusion (transfusion of ≥2 units of packed red blood cells), rebleeding during hospitalization, the need for vascular embolization or surgical treatment after endoscopic hemostasis, or death. As such, 62 patients were classified as having severe DH, while the remaining 211 patients were classified as non-severe DH. We thus investigated 62 cases of severe DH and 211 cases of non-severe DH to evaluate risk factors for severe DH among DH patients in this nested case-control study, using the data-set of our retrospective cohort.1
Factors evaluated included sex (male/female), age ≥70 years, and BMI. BMI was calculated as weight divided by the square of height (kg/m2). Patients were categorized into 3 groups according to the use of oral ATDs (266 low-dose aspirin or other drugs mentioned in the previous report,1 58 anticoagulants warfarin and dabigatran): no ATDs; 1 oral ATD; or ≥2 oral ATDs. Use of NSAIDs (drugs mentioned in the previous report1) was also examined.
Two-group comparisons for lifestyle factors included smoking and alcohol use. We regarded current and previous smoking as representing a positive history of smoking, while only current drinking was defined as positive for alcohol. Other factors examined included history of laparotomy and other background pathology (cerebrovascular disease, ischemic heart disease, hypertension, hyperlipidemia, hyperuricemia, diabetes mellitus, chronic liver disease, and chronic kidney disease). Comorbidity was quantified using the Charlson Risk Index (CRI).7 Two groups were compared: CRI ≤1 and CRI ≥2.
The anatomic distribution of colonic diverticula was divided into 3 groups for comparison: right-sided colonic diverticula in the cecum, ascending colon, or transverse colon; left-sided colonic diverticula in the descending or sigmoid colon; or bilateral colonic diverticula in both sides of the colon. In addition, the identified site of DH was divided into 3 groups for comparison: right-sided DH with bleeding from a diverticulum in the cecum, ascending colon, or transverse colon; left-sided DH with bleeding from a diverticulum in the descending or sigmoid colon; and source of bleeding unknown.
The presence or absence of associated symptoms was also compared, with associated symptoms defined as any of the following: abdominal pain, diarrhea, or symptoms of cerebral hypoperfusion (light-headedness or dizziness or syncope).
This study was approved by the Institutional Review Board of Fukuoka University Chikushi Hospital (IRB No. R15-024) and was conducted in accordance with the Declaration of Helsinki. Since this was a retrospective study, the need to obtain informed consent to participate in the study was waived by the review board.
2. Diagnosis
CS was generally performed on the day of overt bleeding or the day thereafter. Depending on the clinical symptoms and physical examination findings in each patient, CS was performed without pretreatment, or after pretreatment with an enema or bowel-cleansing agent. Olympus colonoscopes (PCF-240AZI, PCF-240AI, CF-240AZI, CF-240ZI, PCF-260AZI, PCF-PQ260I, and CF-260AI; Olympus, Tokyo, Japan) were used for all procedures.
Colonic DH was defined as follows:8
1) Definite Colonic DH (n=207)
(1) Active bleeding observed from a diverticulum and (2) presence of blood clots, erosions, or an exposed vessel near a diverticulum. Either (1) or (2), absence of blood in the terminal ileum on total CS, and no other obvious cause of bleeding.
2) Suspected Colonic DH (n=66)
Obvious bloody stools, no blood in the terminal ileum on CS after bowel preparation, and DH as the only likely cause of bleeding.
3. Cases Requiring Blood Transfusion
During hospitalization, patients with significant bleeding or rebleeding and hemoglobin of 7.0 g/dL or ≤8.0 g/dL or patients with hemoglobin of ≥10.0 g/dL in blood sampled on arrival, but in whom hemoglobin decreased by 5.0 g/dL or more from baseline and hemoglobin of <10 g/dL in blood sampled after several days in hospital because of significant bleeding or rebleeding.
4. Statistical Analysis
All statistical analyses were conducted using the Statistical Analysis System package (SAS Institute, Cary, NC, USA). The chi-square test was used to compare categorical variables between the severe and non-severe DH cases. Unconditional logistic regression was used to compute ORs and their 95% CIs for severe DH. We used age, sex, and other risk factors for severe DH to estimate fully adjusted ORs in relation to factors in the table. Values of P<0.05 were considered to be statistically significant.
RESULTS
The number of patients with DH was increased in association with an increase in the number of cases of severe DH (Fig. 1).
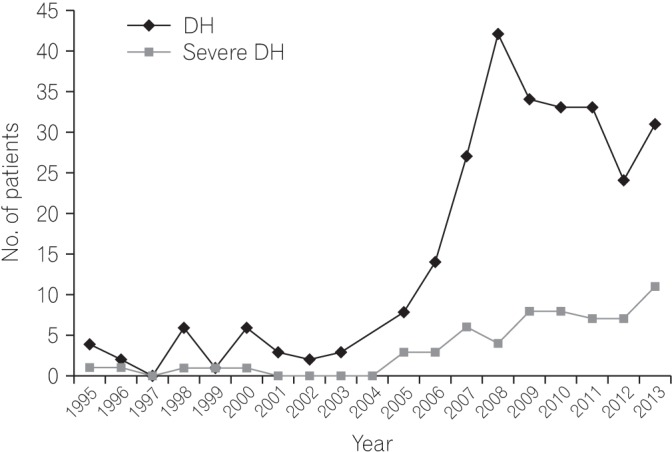
Change in number of patients with colonic diverticular hemorrhage (DH): number of server DH patients (1995 to 2013). The number of patients with DH increased in association with an increase in the number of server DH patients.
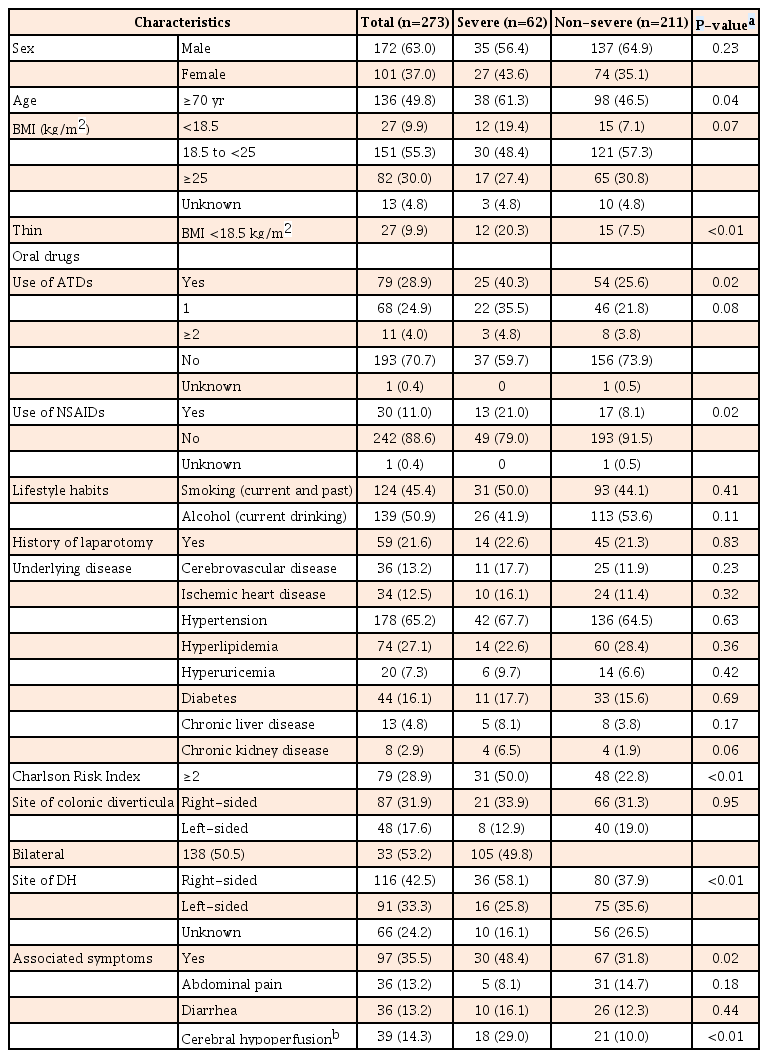
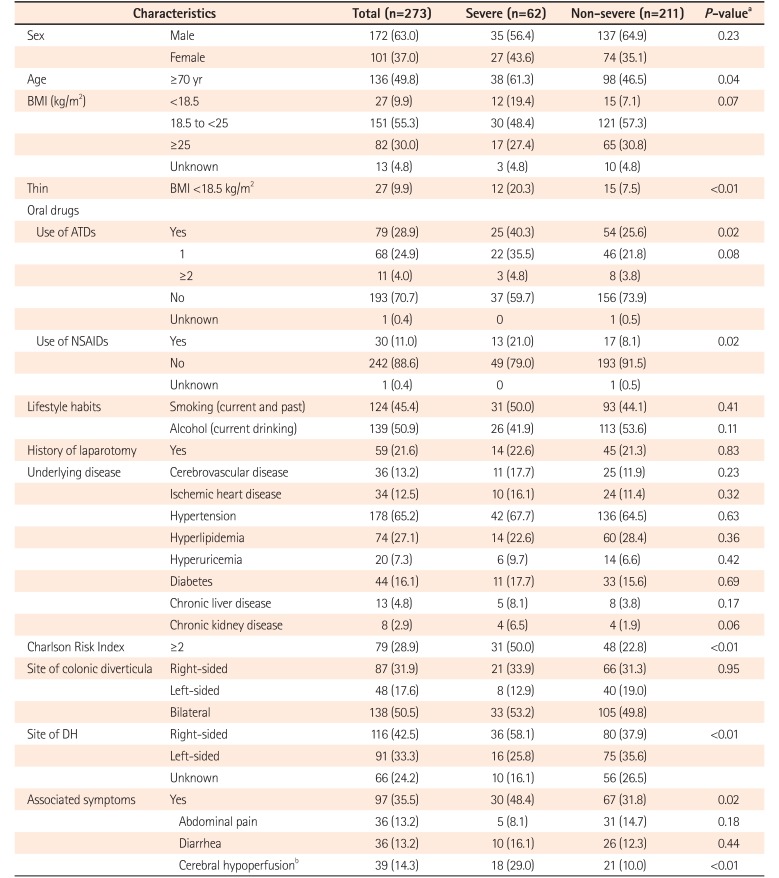
Table 1 shows the characteristics of patients with severe and non-severe DH. Among the 273 patients with DH, 62 (22.7%) met the criteria for severe DH (transfusion: 56 [20.5%]; rebleeding during hospitalization: 11 [4.0%]; vascular embolization: 2 [0.7%]; surgical treatment: 1 [0.3%]; death: 0 [0%]). Regarding transfusion, 43 patients had significant bleeding or rebleeding with hemoglobin of 7.0 g/dL or ≤8.0 g/dL during hospitalization, while 13 patients had hemoglobin of ≥10.0 g/dL in blood sampled on arrival, but their hemoglobin decreased by 5.0 g/dL or more from baseline and hemoglobin of <10 g/dL in blood sampled after several days in hospital because of significant bleeding or rebleeding. In addition, 204 patients (74.7%) showed spontaneous resolution of bleeding without treatment.
In recent years, bilateral colonic diverticular-type DH has been identified as the most common type (Fig. 2).

Change in diverticula distribution (type) in colonic diverticular hemorrhage (DH) (1995 to 2013). Looking at cases of DH by diverticula distribution, bilateral colonic diverticular-type DH has been the most common type since 2007.
Frequencies of age ≥70 years (P=0.04), BMI ≤18.5 kg/m2 (P<0.01), use of ATDs (P=0.02), use of NSAIDs (P<0.01), CRI ≥2 (P<0.01), and right-sided colonic DH (P<0.01) were significantly higher among patients with severe DH compared to those with non-severe DH (Table 1). A higher proportion of severe cases was seen among patients with DH from the right colon, compared to other sites (Fig. 3). In addition, symptoms associated with DH (P=0.02) and symptoms of cerebral hypoperfusion (P<0.01) were more frequent in patients with severe DH than in those with non-severe DH.

Proportion (%) of the site of hemorrhaging diverticulum in severe and non-severe diverticular hemorrhage (DH). In a comparison of severe and non-severe DH by site of hemorrhaging diverticulum, the proportion of DH in the right colon was significantly higher among severe cases
We calculated crude ORs (95% CI) and age- and sex-adjusted ORs (95% CI) to evaluate risk factors for the development of severe DH among patients with DH. Table 2 shows age- and sex-adjusted ORs (95% CI) and fully adjusted ORs (95% CI) from multivariate analysis after adjustment for sex, age, thin build (BMI <18.5 kg/m2), ATDs, NSAIDs, CRI ≥2, right-sided colonic DH, and symptoms of cerebral hypoperfusion. Even after controlling for sex, age, and all factors described in the table, oral NSAIDs (OR, 2.801; 95% CI, 1.164–6.742; P=0.02), CRI ≥2 (OR, 3.336; 95% CI, 1.154–7.353; P<0.01), right-sided colonic DH (OR, 3.873; 95% CI, 1.554–9.653; P<0.01), and symptoms of cerebral hypoperfusion (OR, 2.926; 95% CI, 1.310–6.535; P<0.01) remained as risk factors for severe DH, while thin build (BMI <18.5 kg/m2) and use of ATDs did not.
DISCUSSION
Colonic DH has recently been reported as the most common cause of LGIB in Japan.26910 We previously reported that the incidence of DH has increased since 2000.1 The present study demonstrated that the number of severe DH patients increased in accordance with the absolute increase in the total number of DH patients.
Bleeding only occurs in 2% to 5% of patients during the natural history of diverticulosis, and most cases are relatively mild, resolving spontaneously in ≥75%–93% of cases.111213 However, rebleeding has been reported in 13% to 38% of cases where bleeding does occur.68131415
Previous case-control studies in Japan identified several risk factors for DH: the use of ATDs and NSAIDs, hypertension, arteriosclerotic diseases such as ischemic heart disease, diabetes mellitus, and chronic kidney disease, age ≥70 years, and cluster-type diverticulosis. Other reports confirmed these as risk factors.16910111617
Nagata et al.14 reported gradually increasing rates of long-term rebleeding of 3.1%, 19%, 27%, and 38% after 1, 6, 12, and 24 months of follow-up for colonic diverticulosis, respectively, and also reported continued use of oral NSAIDs as a risk factor for rebleeding. Other studies have also reported a 3- to 6-fold increase in the risk of rebleeding in colonic diverticulosis patients taking NSAIDs.1015 While many reports have examined risks for DH, factors that increase DH severity have seldom been investigated.
Our study aimed to establish severity criteria for DH. We then extracted risk factors for severe DH with an aim to identify and classify these risk factors so that severe DH can be predicted and standardized methods of treatment developed. After comparing patients with severe and non-severe DH, we identified 4 risk factors for increased severity: the use of oral NSAIDs; CRI ≥2; right-sided colonic DH; and symptoms of cerebral hypoperfusion. Besides being a risk factor for both DH and rebleeding, the use of NSAIDs also appears to be a risk factor for severe DH. The mechanisms underlying DH have yet to be fully elucidated. However, colonic diverticula develop at sites where the vasa recta penetrate the large intestinal wall when intestinal pressure increases. Blood vessels in the diverticula are separated from the bowel lumen only by the mucosa and are easily injured. With repeated mechanical stimuli, intimal thickening of the vasa recta occurs, often with thinning of the media. These changes can cause segmental weakening of the vasa recta, which may lead to arterial hemorrhage in the bowel lumen.18
Arteriosclerosis also plays a role in the pathogenesis, leading to rupture of blood vessels in diverticula.1920 Blood vessel fragility due to arteriosclerosis may result in bleeding. Patients with colonic diverticulosis and patients who develop DH are generally elderly.21 Moreover, the frequency of arteriosclerosis increases with aging, so the possibility of a pathogenic role of arteriosclerosis in DH warrants consideration. However, not all patients with diverticulosis experience bleeding; indeed, most remain asymptomatic. Therefore, in addition to arteriosclerosis, other factors increase the fragility of blood vessels in diverticula.
Multivariate analysis in the present study was performed with adjustment for factors including sex and age, so analysis was performed independently of arteriosclerosis. The results identified NSAIDs as a significant risk factor, suggesting that NSAIDs have a synergistic effect on the injury of blood vessels in diverticula. Most NSAIDs inhibit PG synthesis, which disrupts the microcirculation and inhibits platelet aggregation. Thus, in patients with diverticulosis, NSAIDs can lead to bleeding, including severe DH.17 On the other hand, our study did not find an association between ATDs and severe DH. This suggests a weaker adverse effect of ATDs on DH severity compared with NSAIDs.
The potential reasons for right-sided colonic DH representing a risk factor for severe DH factor must be considered. Right-sided diverticula are often larger than left-sided diverticula, so the vasa recta are more easily injured. In addition, because of the relatively thinner intestinal wall, the amount of bleeding from right-sided colonic diverticulosis is often greater, and the rate of rebleeding is also higher.1922 Furthermore, because of the longer distance from the anus, bleeding from right-sided colonic diverticulosis is often detected later than bleeding from left-sided colonic diverticulosis, thus increasing DH severity. NSAID-induced colopathy is also reported to occur more commonly in the terminal ileum and right colon. Due to increasing awareness of the upper gastrointestinal (GI) side effects of NSAIDs, enteric-coated and slow-release formulations have been introduced.23 With such formulations, intestinal mucosa in the lower GI tract is directly subjected to free NSAIDs. The fact that the lesions have only been described in the right colon, where the cecum acts as a reservoir and the mucosa is thus subjected to the NSAIDs for a longer time, may support this hypothesis.242526 Consequently, use of NSAIDs and bleeding from right-sided colonic diverticula are thought to be strongly associated with severity of DH.
About 50% of patients with colonic DH in our study were ≥70 years old and had a high CRI, which correlates strongly with arteriosclerotic diseases; this was a risk factor for increased DH severity. Although no individual underlying disease alone was identified as a risk factor for severe DH, the CRI assigns scores to conditions including multiple arteriosclerotic diseases. Moreover, the same disease, depending on severity, may yield different scores. The CRI is therefore thought to offer a useful index of arteriosclerosis severity and multiple comorbid conditions.
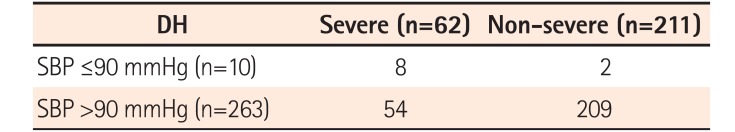
In addition, our study identified symptoms of cerebral hypoperfusion (light-headedness or dizziness or syncope) as a risk factor for DH severity. Lee et al.27 also included a systolic blood pressure (SBP) of ≤90 mmHg as a criterion for severe DH. This can signify an acute fall in blood pressure due to massive bleeding. In our study, symptoms of cerebral hypoperfusion such as light-headedness, dizziness, and syncope suggested acute massive bleeding, necessitating urgent treatment, including blood transfusions. Endoscopic treatment, which may avoid the need for emergency surgery and prevent further bleeding,828 should be instituted aggressively for patients in a stable general condition. In this study, as shown in Table 3, only 10 patients had a SBP of ≤90 mmHg. Moreover, since patients in shock or with unstable vital signs could not undergo endoscopic examination, they were not included in the study. However, the relationship between the severity of DH and SBP ≤90 mmHg had a low sensitivity of 12.9%, and was thus omitted from the investigation items in the analysis despite its high specificity (99.1%).
Nagata et al.29 described female sex, warfarin, chronic kidney disease, age ≥70 years, and NSAID use as risk factors for severity in DH (defined based on the need for transfusion and long-term hospitalization). Aoki et al.30 presented 8 factors (CRI ≥2, syncope, absence of diarrhea, absence of abdominal tenderness, use of NSAIDs, use of non-aspirin antiplatelet drugs, blood pressure ≤100 mmHg, and albumin <3.0 g/dL) as risk factors for severity in LGIB including colonic DH (continued bleeding during the first 24 hours [transfusion of ≥2 units of packed red blood cells and/or decrease in hematocrit of ≥20%], and/or recurrent bleeding after initial CS [rectal bleeding accompanied by further decrease in hematocrit of ≥20% and/or additional blood transfusions]). Both studies2930 showed points in common with the present study.
Our study examined DH patients who underwent CS for LGIB over a 19-year period, including detailed information about underlying diseases and medications. The investigation included a relatively large cohort of 273 patients with colonic DH for comparison and analysis. We also compared the baseline data of patients with severe and non-severe DH patients among all subjects in this cohort. We were able to understand the changes with time and incidence over a long period in patients with severe DH.
There are some limitations to our study. One was the retrospective nature of the study design, and another was the fact that only a single institution was involved. In addition, multi-detector row CT was not used at the initial visit to evaluate the site of bleeding, so accurate determinations could not be made in all cases. Furthermore, this study included ex-smokers as smokers. However, a patient who has not smoked for more than 10 years may not have the same risk as a current smoker. However, we were unable to collect more detailed information about smoking habits, due to the retrospective nature of the study. We also did not evaluate general arteriosclerosis via carotid artery ultrasonography. We checked charts and reviewed the patients' heart rate at the time of initial visit. But details of the heart rate were not described in more than 40 cases out of 273 cases. Therefore, this item was not able to be added as risk factor in this study.
The strengths of this study are 2-fold: Firstly, there was no selection bias because we selected all severe and all non-severe patients among the DH patients in our retrospective cohort. Secondly, there was little information bias in this study because we used information from medical records before and at the diagnosis of DH.
In conclusion, patients with multiple arteriosclerosis-related diseases and/or those taking NSAIDs were more likely to have severe DH, including a higher risk of rebleeding and a need for blood transfusions. Measures to prevent rebleeding such as stopping NSAIDs may be necessary in these patients. Patients showing symptoms of cerebral hypoperfusion or bleeding from right-sided diverticulosis are also at risk of severe DH. These patients require urgent treatment, including endoscopy to identify the source of bleeding. These factors thus should be kept in mind when treating patients with DH.
ACKNOWLEDGEMENTS
We wish to thank Mr. Matt Morgan of Forte, Inc. for providing advice on the use of English in the manuscript.
Notes
FINANCIAL SUPPORT: The authors received no financial support for the research, authorship, and/or publication of this article.
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION: Kinjo K collected and analyzed the data, and drafted the manuscript; Matsui T designed and supervised the study; Washio M performed the statistical analysis; Hisabe T, Ishihara H, Kojima T, Chuman K, Yasukawa S, Beppu T, Koga A, Ishikawa S, Kishi M, Takatsu N, Hirai F, Yao K, Ueki T performed the colonoscopy and revised the manuscript for important intellectual content; all authors have read and approved the final version to be published.