 |
 |
- Search
| Intest Res > Volume 12(3); 2014 > Article |
|
Abstract
Amyloidosis is characterized by the abnormal deposition of extracellular amyloid fibrils. Cases involving amyloid light-chain amyloidosis in the small intestine have been reported infrequently in Korea. Here, we report a case of localized light chain protein amyloidosis in the small intestine. Esophagogastroduodenoscopy, push enteroscopy, and capsule endoscopy revealed submucosal tumor-like lesions, multiple shallow ulcers, and several erosions in the distal duodenum and jejunum. An endoscopic biopsy established the diagnosis of amyloidosis. In through an immunohistochemical analysis, the presence of lambda light chain protein was detected. The patient had no evidence of an underlying clonal plasma cell disorder or additional organ involvement. Therefore, we concluded that the patient had localized amyloidosis of the small intestine.
Amyloidosis is a disorder characterized by deposition of amyloids or fibrous proteins consisting of low molecules in the extracellular space of tissues due to abnormal protein metabolism. Amyloid deposits can occur in various organs or tissues with widely varying clinical features. Although amyloidosis in the gastrointestinal tract is not uncommon, it is difficult to diagnose due to inconsistent clinical features and endoscopic findings. In addition, amyloidosis localized primarily in the small intestine in the absence of underlying disease has rarely been observed. We report here a case of primary amyloidosis only in the small intestine in the absence of any other disease, as well as a review of relevant literature.
A 62-year-old man visited our hospital with complaints of epigastric pain, nausea, and constipation which had started several years ago. According to the patient, these symptoms began to worsen 6 months ago. Abdominal CT conducted in another hospital detected an enlarged lymph node that was found to be improved in a follow-up examination performed 6 months ago. Except for this finding, the patient's history was unremarkable. Regarding the patient's vital signs, his blood pressure, pulse rate, respiratory rate and body temperature were 120/80 mmHg, 70 beats/min, 16 breaths/min, and 36.7Ōäā, respectively. A peripheral blood test showed leukocyte, hemoglobin, and platelet levels of 8,300/mm3 (granulocyte, 64%; lymphocyte, 26%; eosinophil, 1%), 13.3 g/dL, and 236,000/mm3, respectively. A serological examination revealed that levels of albumin, total bilirubin, AST/ALT, ALP, and BUN/Cr were 4.0 g/dL, 0.33 mg/dL, 23/23 IU/L, 64 IU/L, and 7.0/11.6 mg/dL, respectively. Urinalysis did not reveal signs of proteinuria.
No abnormal upper gastrointestinal endoscopy findings were observed in the esophagus and the stomach, however, elevated lesions with central erosion were found on the third portion of the duodenum (Fig. 1). Colonoscopy did not detect any abnormalities. Abdominal CT was conducted to assess the previously observed abdominal lymphadenopathy. With the exception of an unclear, hazy appearance of the ventral mesoderm, no abnormalities were detected (Fig. 2). Push enteroscopy to examine lesions in the duodenum detected multiple shallow ulcers with various degrees of elevation and color from the duodenum to the jejunum (Fig. 3). Endoscopic ultrasonography of the elevated lesions and shallow ulcers revealed hypoechoic thickening of the mucous and the submucosal layers (Fig. 4), and capsule endoscopy detected multiple shallow ulcers limited to the duodenum and the jejunum (Fig. 5).
Histological examination of the duodenum and the jejunum revealed amorphous, pink deposits in the lamina propria (Fig. 6A), and positive Congo red staining with salmon-colored deposits (Fig. 6B). Apple-green birefringence was evident using polarizing microscopy (Fig. 6C), and the patient was diagnosed with amyloidosis. According to the results of immunohistochemical tests, deposits of amyloid were positive for amyloid P and amyloid lambda, but negative for amyloid A. Therefore, the deposits of amyloid were considered to be indicative of amyloid light-chain (AL) amyloidosis. Additional biopsies of the esophagus, stomach, and terminal ileum were conducted and no abnormalities were detected.
A small bowel series to assess the amyloidosis invasion area did not show any abnormalities. In immunoserological tests, the rheumatic factor, antinuclear antibody, and anti CCP results were negative, and the IgG level was normal at 1,022 mg/dL (reference range 408-1,788 mg/dL). Immunoelectrophoresis to detect any abnormalities of plasma cells showed that the free kappa light chain level was normal at 8.23 mg/L (reference range 3.3-19.4 mg/L). Although the lambda light chain level was slightly high at 29.58 mg/L (reference range 5.71-26.3 mg/L), the kappa/lambda ratio was normal at 0.28 (reference range 0.26-1.65).
Protein electrophoresis and immunoelectrophoresis of serum and urine found no abnormalities, and a bone marrow biopsy also showed no abnormalities. Additionally, echocardiography did not detect any abnormalities and PET did not find abnormal 18F-fluorodeoxyglucose (FDG) uptake.
Based on these collective findings, the patient was diagnosed with localized AL amyloidosis in the small intestine. He was followed-up for 12 months as an outpatient prescribed 7.5 mg/day prednisolone and had no complications.
Depending on the biochemical characteristics of the precursor proteins forming the amyloids, amyloidosis can be categorized into primary amyloidosis (AL amyloidosis) associated with immunoglobulin light chains, secondary amyloidosis (AA amyloidosis) due to the presence of amyloid A proteins associated with chronic inflammation, and dialysis-related amyloidosis characterized by the deposition of beta-2-microglobulin associated with dialysis. Additionally, there are other categories of amyloidosis, including senile systemic amyloidosis related to transthyretin, and genetic amyloidosis.1
Although invasion of the gastrointestinal tract was reported in 60% of AA amyloidosis cases,2 it was found to be rare in cases of AL amyloidosis with symptoms (1%).3 The invasion of the small intestine from the gastrointestinal tract was frequently observed,4 but has been regarded as difficult to diagnose due to the wide variation in symptoms observed based on the sites that have been invaded.
Amyloidosis in the small intestine can be accompanied by various symptoms. Deposition on vascular walls can lead to thickening, and deposition in blood vessels can provoke intestinal ischemia or mesenteric infarction. Furthermore, invasion in the muscularis mucosae can trigger gastrointestinal motility disorder. The main clinical symptoms of amyloidosis are diarrhea, steatorrhea, protein loss and malabsorption, and hemorrhage. Additional symptoms include mesenteric infarction, as well as ileus and intestinal perforation. Although liver invasion is common, symptoms are generally mild and similar to those observed for hepatomegaly. An increased alkaline phosphatase level is most frequently observed in liver function test.5
Amyloidosis in the gastrointestinal tract can be associated with various endoscopic findings. Elevated lesions or significant mucosal folds are common for AL amyloidosis and are attributable to invasion into the mucous or the submucosal layer, or the muscularis propria. For AA amyloidosis, the deposition of granules results in fine granular lesions, or erosion or vulnerability of the mucous layer. These differences result in constipation, intestinal obstruction, and intestinal pseudo-obstruction as common symptoms of AL amyloidosis, and diarrhea or malabsorption as common symptoms of AA amyloidosis.6 The patient in this case also showed various endoscopic findings ranging from elevated to erosive lesions.
To confirm amyloidosis, a direct biopsy of invaded lesions or a biopsy of subcutaneous fats or the bone marrow should be conducted. Decisions regarding the sites selected to obtain biopsy specimens should depend on accessibility to lesions and complications such as hemorrhage.
Although biopsies of the rectum or subcutaneous fats are performed frequently to avoid adverse events, biopsies of the lesions in the mucous layer are necessary due to a high level of sensitivity for gastrointestinal amyloidosis.7 As amyloids are well-deposited on the vascular walls of the lamina propria, biopsy specimens should include sufficient amounts of the lamina propria. In this case, the patient had lesions in the mucosal layer of the small intestine and also underwent biopsies of the esophagus, stomach, and the small intestine. Additional biopsies of other organs, such as the liver, were not conducted due to a lack of abnormalities in liver function tests and abdominal CT.
Endoscopic ultrasonography and narrow band imaging can be helpful in diagnosing gastrointestinal amyloidosis. As seen in the patient in this case, thickening of the mucosal and the submucosal layers, as well as top layer loss, have been observed using endoscopic ultrasonography in patients with amyloidosis.8,9 Additionally, ulcers surrounded by a greenish gray mucosal layer without an abnormal blood pattern have been found using narrow band imaging.9 When amyloids invade the small intestine, lesions are observed most clearly through radiological imaging.10 In abdominal CT, thickening or distention of the tract may be seen, however, abnormalities of the mesentery have rarely been observed.11 Although previous studies have reported that increased FDG uptake during PET was observed for localized amyloidosis and primary amyloidosis,12,13 others, including the present case, have not found such an increase for localized small intestine amyloidosis. Therefore, prospective studies are needed.14,15
If amyloidosis is diagnosed, immunohistochemical tests or immunofluorescence microscopy should be performed to determine the subtype. Positive kappa or lambda light chain staining are indicative of primary amyloidosis and positive amyloid A staining indicates secondary amyloidosis. As all subtypes of amyloidosis show a positive result for amyloid P staining, this stain may be helpful in diagnosing amyloidosis. As the patient in this case showed positive results in lambda light chain and amyloid P staining, he was diagnosed with primary amyloidosis.
Primary systemic amyloidosis can only be diagnosed when a general symptom related to amyloids, such as amyloids observed using Congo red staining or electron microscopy, or the formation of light chains and monoclonal plasma cell proliferative disorder, are present. Furthermore, monoclonal plasma cell proliferation disorder can be confirmed when either M proteins are detected in the serum or urine, an abnormal free light chain ratio is observed, or when monoclonal plasma cells are observed during a bone marrow examination.16,17 Conversely, most patients with localized amyloidosis do not show monoclonal Ig and amyloid infiltration limited to a single organ. The patient in this case also did not have monoclonal plasma cell proliferation disorder, and his lesions were limited to the small intestine.
The therapeutic goal in the treatment of primary amyloidosis is to cure an underlying disease and to control symptoms. Steroids or chemical therapy to reduce the formation of Ig light chains are often used. In some cases, bone marrow transplantation after high-dose chemical therapy is conducted. However, light chains shown in localized amyloidosis patients are limited to lesions.18 Therefore, systematic treatment is not needed and the prognosis is often quite good.
Localized small intestine amyloidosis can be difficult to diagnose due to a low incidence rate, non-specific symptoms, and failure to detect abnormalities using radiological imaging. As shown in this case, a close examination of the small intestine during upper gastrointestinal endoscopy or colonoscopy is necessary. If amyloidosis in the small intestine is not suspected, the necessary tests should be performed to either actively rule it out or to diagnose and differentiate it.
References
1. Ebert EC, Nagar M. Gastrointestinal manifestations of amyloidosis. Am J Gastroenterol 2008;103:776-787.PMID: 18076735.


2. Okuda Y, Takasugi K, Oyama T, Onuma M, Oyama H. Amyloidosis in rheumatoid arthritis--clinical study of 124 histologically proven cases. Ryumachi 1994;34:939-946.PMID: 7863383.

3. Menke DM, Kyle RA, Fleming CR, Wolfe JT 3rd, Kurtin PJ, Oldenburg WA. Symptomatic gastric amyloidosis in patients with primary systemic amyloidosis. Mayo Clin Proc 1993;68:763-767.PMID: 8331978.


4. Tada S, Iida M, Iwashita A, et al. Endoscopic and biopsy findings of the upper digestive tract in patients with amyloidosis. Gastrointest Endosc 1990;36:10-14.PMID: 2311879.


5. Park MA, Mueller PS, Kyle RA, Larson DR, Plevak MF, Gertz MA. Primary (AL) hepatic amyloidosis: clinical features and natural history in 98 patients. Medicine (Baltimore) 2003;82:291-298.PMID: 14530778.


6. Tada S, Iida M, Yao T, Kawakubo K, Okada M, Fujishima M. Endoscopic features in amyloidosis of the small intestine: clinical and morphologic differences between chemical types of amyloid protein. Gastrointest Endosc 1994;40:45-50.PMID: 8163134.


7. Breedveld FC, Markusse HM, MacFarlane JD. Subcutaneous fat biopsy in the diagnosis of amyloidosis secondary to chronic arthritis. Clin Exp Rheumatol 1989;7:407-410.PMID: 2591113.

8. Grape T, Johansson GW, Eriksson M, Toth E, Thorlacius H. Primary gastroduodenal amyloidosis. Endoscopy 2011;43:E288PMID: 21915830.



9. Sawada T, Adachi Y, Akino K, et al. Endoscopic features of primary amyloidosis of the stomach. Endoscopy 2012;44(Suppl 2): E275-E276.PMID: 22814919.



10. Kim SH, Han JK, Lee KH, et al. Abdominal amyloidosis: spectrum of radiological findings. Clin Radiol 2003;58:610-620.PMID: 12887954.


11. Araoz PA, Batts KP, MacCarty RL. Amyloidosis of the alimentary canal: radiologic-pathologic correlation of CT findings. Abdom Imaging 2000;25:38-44.PMID: 10652919.


12. Mekinian A, Jaccard A, Soussan M, et al. 18F-FDG PET/CT in patients with amyloid light-chain amyloidosis: case-series and literature review. Amyloid 2012;19:94-98.PMID: 22587492.


13. Glaudemans AW, Slart RH, Noordzij W, Dierckx RA, Hazenberg BP. Utility of 18F-FDG PET(/CT) in patients with systemic and localized amyloidosis. Eur J Nucl Med Mol Imaging 2013;40:1095-1101.PMID: 23474745.


14. Tang YH, Wu YW, Zong J, Wang JC, Miao F. Imaging features of small intestinal amyloidosis: a case report. Abdom Imaging 2011;36:694-697.PMID: 21221573.


15. Mainenti PP, Segreto S, Mancini M, et al. Intestinal amyloidosis: two cases with different patterns of clinical and imaging presentation. World J Gastroenterol 2010;16:2566-2570.PMID: 20503459.



16. Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 2009;23:3-9.PMID: 18971951.



17. Rajkumar SV. Multiple myeloma: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol 2011;86:57-65.PMID: 21181954.


18. Hamidi Asl K, Liepnieks JJ, Nakamura M, Benson MD. Organ-specific (localized) synthesis of Ig light chain amyloid. J Immunol 1999;162:5556-5560.PMID: 10228037.



Fig.┬Ā1
Upper endoscopic findings. Endoscopy revealed an elevated lesion with central erosion on the third portion of the duodenum.
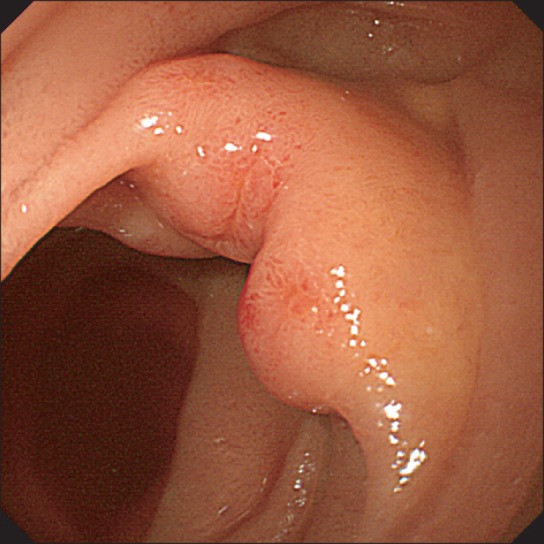
Fig.┬Ā2
Abdominal CT findings. Abdominal CT scan revealed haziness in the mesentery (arrows) without abnormal enhancement of wall thickening and dilatation of the small bowel.

Fig.┬Ā3
Push enteroscopic findings. Endoscopy revealed (A) multiple shallow ulcers (white arrows) with broad bases and (B) discoloration of the jejunum (black arrow).

Fig.┬Ā4
Endoscopic ultrasonography (EUS) findings. EUS revealed hypoechoic thickening of the muscularis mucosa on (A) a flat lesion with a shallow ulcer and (B) an elevated lesion. Submucosal thickening was present on the elevated lesion (C).

Fig.┬Ā5
Capsule endoscopy (CE) findings. CE revealed (A) multiple shallow ulcers and (B) whitish plaque-like infiltration in the jejunum.
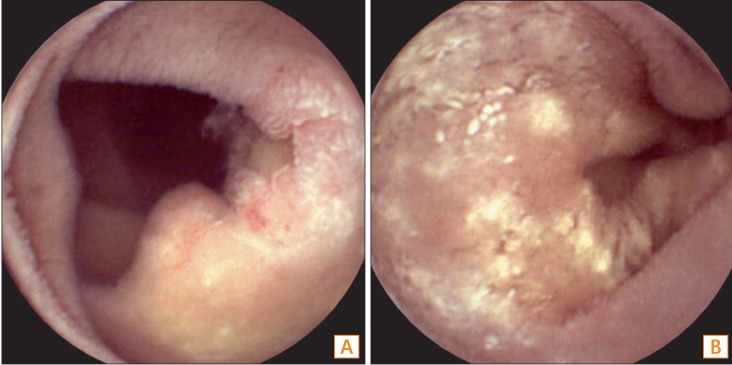
Fig.┬Ā6
Histopathologic analysis. Microscopic findings revealed (A) the presence of amorphous eosinophilic material deposits in the lamina propria (├Ś100), (B) salmon-colored deposits of amyloid using Congo red staining (├Ś100), and (C) the apple-green color birefringence of the deposits using polarizing microscopy (├Ś100).

- TOOLS






