 |
 |
- Search
| Intest Res > Volume 15(4); 2017 > Article |
|
Abstract
Background/Aims
Patients with small bowel strictures have varied etiologies, including malignancy. Little data are available on the demographic profiles and etiologies of small bowel strictures in patients who undergo surgery because of intestinal obstruction but do not have a definitive pre-operative diagnosis.
Methods
Retrospective data were analyzed for all patients operated between January 2000 and October 2014 for small bowel strictures without mass lesions and a definite diagnosis after imaging and endoscopic examinations. Demographic parameters, imaging, endoscopic, and histological data were extracted from the medical records. Univariate and multivariate analyses were conducted to identify factors that could differentiate between intestinal tuberculosis (ITB) and Crohn's disease (CD) and between malignant and benign strictures.
Results
Of the 7,425 reviewed medical records, 89 met the inclusion criteria. The most common site of strictures was the proximal small intestine (41.5%). The most common histological diagnoses in patients with small bowel strictures were ITB (26.9%), CD (23.5%), non-specific strictures (20.2%), malignancy (15.5%), ischemia (10.1%), and other complications (3.4%). Patients with malignant strictures were older than patients with benign etiologies (47.6±15.9 years vs. 37.4±16.4 years, P=0.03) and age >50 years had a specificity for malignant etiology of 80%. Only 7.1% of the patients with malignant strictures had more than 1 stricture and 64% had proximally located strictures. Diarrhea was the only factor that predicted the diagnosis of CD 6.5 (95% confidence interval, 1.10-38.25; P=0.038) compared with the diagnosis of ITB.
In gastrointestinal practice, patients often present with symptoms of partial bowel obstruction. The examination of these patients involves cross-sectional imaging, which frequently shows small bowel strictures. These patients may have clinical features that indicate a particular disease or concomitant imaging findings such as mass lesions or adjacent lymph nodes, which may lead to a diagnosis. However, other diagnostic clues are rare. The presence of single or multiple small bowel strictures without mass lesions, colonic involvement, and mesenteric or retroperitoneal lymphadenopathy may be due to diverse causes. Only a few studies evaluated the etiology of small bowel strictures and suggested other causes for this condition, including NSAIDs-related diaphragmatic disease, ischemic stricture, and malignancy.1,2,3,4 However, in the absence of the intake of NSAIDs, most clinicians limit the differentiation to CD and intestinal tuberculosis (ITB). ITB is an important cause of strictures, especially in endemic countries such as India. ITB occurs mainly in the terminal ileum and ileo-cecal junction but can also occur in the proximal small bowel. CD is an important cause of small bowel strictures and is being diagnosed more often even in countries endemic for tuberculosis.5,6 Because of the absence of diagnostic clues in small bowel strictures, patients are often treated with anti-tubercular therapy (ATT), which has adverse effects, or steroids in cases of inadequate response to ATT. In the absence of such data, the treating clinician is confronted with a dilemma: to choose between surgical confirmation of diagnosis or empirical medical therapy for common causes, including ITB and CD. Therefore, it is important to differentiate common causes of strictures (such as ITB and CD) from rare etiologies, especially malignancy, because this differentiation can lower the threshold for surgical resection. Therefore, we retrospectively analyzed the records of all patients who underwent surgery for single or multiple small bowel strictures at a tertiary care center in northern India over the past 15 years and did not have a definitive preoperative diagnosis.
The medical records of 7,425 patients who were operated from January 2000 to December 2014 in the Department of Gastrointestinal Surgery, All India Institute of Medical Sciences, New Delhi, were screened for diagnosis, imaging findings, and operative procedures. Patients whose case records met the following criteria were included in study: (1) those with preoperative findings of small bowel stricture on any imaging examination, including barium meal follow-through, contrast enhanced CT, CT enterography, or MR enterography; (2) age >12 years. We excluded patients in whom there was: (1) a concomitant growth or mass lesion either on imaging or endoscopic examination; (2) a definitive preoperative histological diagnosis.
Eighty-nine patients met the study criteria and their data were included in the analysis. Data were extracted to a specifically designed proforma and then transferred to an Excel sheet. Baseline demographic profile, clinical symptoms, previous history of receiving antitubercular drugs, routine blood investigations, imaging findings, endoscopic findings, intra-operative findings, and final histological diagnosis were extracted from the case records and analyzed. The site of the stricture was identified by imaging and classified as terminal ileal; terminal ileal with ileo-cecal junction, cecal, or ascending colon involvement; ileal (including strictures located in the ileum proximal to the terminal ileum); and proximal small bowel (including strictures in the duodenum and jejunum). All biopsy specimens were reviewed by the same pathologist for confirmation of the histological diagnosis.
Informed consent for this study was waived.
Statistical analysis was done using software SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). All qualitative variables were expressed as percentages, whereas quantitative variables were expressed as mean±SD. The categorical characteristics were compared using the chi-square test. Normality was tested for each continuous parameter and the statistical test was performed accordingly. The factors that could differentiate ITB from CD and malignant from non-malignant strictures were identified using univariate logistic regression analysis followed by multivariate logistic regression analysis.
Eighty-nine patients were included in the analysis. The median age of the patients was 35 years (range, 13-79 years); 42 patients were men and 47 were women. The median duration of symptoms was 12 months (interquartile range, 3-30 months). The predominant symptom was abdominal pain (91.0%) followed by weight loss (77.5%). Other observed symptoms were intestinal obstruction (43.8%), fever (34.8%), diarrhea (18.0%), and gastrointestinal bleeding (7.8%). Hypoalbuminemia (serum albumin <3.5 mg/dL) was present in 52.8% of the patients. In addition, 38 patients (42.7%) had a previous history of receiving ATT.
ITB (26.9%) was the most common histological diagnosis (Fig. 1), followed by CD (23.5%) and non-specific strictures (20.2%). Malignancy accounted for 15.5% (n=14) of the strictures, including 8 cases of adenocarcinoma, 4 cases of lymphoma, 1 case of neuroendocrine tumor, and 1 case of gastrointestinal stromal tumor. Ischemic strictures were present in 9 patients (10.1%). Other etiologies included 2 cases of eosinophilic enteritis and 1 case of autoimmune enteritis (Table 1). The most common site of strictures was the proximal small intestine (41.5%). The stricture sites according to etiology are shown in Table 1.
Table 2 compares the characteristics of benign and malignant strictures. The mean age of the patients with malignant strictures was significantly higher than that of patients with non-malignant strictures (47.6±15.9 years vs. 37.4±16.4 years, P=0.03). Diarrhea was not observed in patients with malignant strictures although the incidence in this group was not significantly different from that of patients with benign strictures (0% vs. 21%, P=0.06). The rate of abdominal pain was non-significantly lower in patients with malignant strictures (79% vs. 93%, P=0.08). Proximal small bowel involvement was non-significantly higher in patients with malignant strictures (64% vs. 37%, P=0.06). Of the 14 patients with malignant strictures, only 1 patient (7%) had more than 1 stricture compared with 17% of the patients (13/75) with non-malignant strictures. Age was the only significant factor in the univariate analysis, and therefore a receiver operating characteristic (ROC) curve (area under the ROC curve, 0.70) was plotted to define a cutoff for age to predict malignant strictures. A cutoff of 49.5 years had a sensitivity and specificity of 57% and 80%, respectively, for malignant stricture (Fig. 2).
Logistic regression analysis was done to identify factors that could differentiate between ITB and CD (Table 3). Diarrhea and hypoalbuminemia were significant factors in the univariate analysis whereas diarrhea was the only independent factor that could help differentiate CD from ITB in the multivariate analysis (Table 3).
Patients presenting with recurrent abdominal pain or intestinal obstruction often have evidence of isolated small bowel strictures on imaging. Of these, many patients have classic clinical or imaging features that allow a definite diagnosis. However, dilemma arises in patients whose symptoms do not allow a definite diagnosis, especially in patients without imaging findings such as mesenteric changes and mass or lymph nodes. In these situations, other than differentiating CD from ITB (which are the most common etiologies), the differentiation between malignant and benign strictures, especially in older patients, is equally important because the threshold for operative intervention is low in malignant strictures. To the best of our knowledge, only a few studies addressed this topic and the present study tries to solve this clinical dilemma. We analyzed the data of 89 patients who underwent surgery for small bowel strictures (without definite preoperative diagnosis) at our center. The most common site of stricture was the proximal small bowel (41.5%) followed by the terminal ileum (28.1%). As expected, the most common final histological diagnosis was ITB (26.9%) followed by CD (23.5%). Therefore, ITB and CD accounted for approximately 50% of the undiagnosed cases of small bowel strictures. The number of diagnoses of CD was similar to that of ITB, indicating that the incidence of IBD is increasing in India.5,6 Other than diarrhea (which is a common symptom),7,8 there were no significant differences in the clinical, imaging, or operative findings between CD and ITB patients. It is of interest that neither the location nor the number of strictures could help differentiate between these 2 diseases. Therefore, in undiagnosed cases of small bowel strictures, the differentiation between CD and ITB is difficult, and surgery should be performed early in cases in which the patient does not respond to ATT.
The most important finding in the present study was that, although ITB and CD were the most common diagnoses, they accounted for only 50% of the cases. Among the remaining patients, malignancy was the most common cause, present in approximately 16% of the cases, and included adenocarcinoma, lymphoma, gastrointestinal stromal tumor, and neuroendocrine tumor. We found that age >50 years had a specificity for malignant etiology of 80%. Moreover, only 1 patient with malignant stricture had more than 1 stricture preoperatively, and 64% of the patients with malignant strictures had proximal small bowel involvement. Among the patients aged >50 years with a single stricture and proximal bowel involvement, 44% had a malignant etiology. Therefore, the threshold for surgery is low in patients aged >50 years with a single undiagnosed isolated stricture in the proximal small bowel.
The second most common etiology after ITB/CD and malignant stricture was ischemic stricture, present in approximately 10% of the patients. Ischemic strictures are often overlooked as a cause of intestinal obstruction and are rarely suspected in clinical cases. Ischemic strictures showed a predilection for the proximal segments of the small intestine considering that 7 of 9 patients had strictures in this region (Table 1). Therefore, ischemic strictures should be suspected, particularly in patients who do not respond to empirical treatment of CD or ITB. Furthermore, approximately 20% of the study patients had a non-specific histology and thus a definitive diagnosis could not be reached even after surgical resection.
Our study has limitations. First, the nature of the study was retrospective and therefore the reliability of the extracted information may be questioned. Second, the fact that our center is a tertiary care referral hospital with special interest in IBD may produce a referral bias. Third, our study initiated in the year 2000. In this respect, endoscopic procedures for small bowel lesions such as double balloon/single balloon enteroscopy were not widely available in India then. Therefore, many patients with small bowel strictures remained undiagnosed and received empirical treatment. Furthermore, these procedures may not be advisable to many of these patients because they frequently present with symptoms of bowel obstruction and the diagnosis of these symptoms is technically difficult in the presence of strictures. For this reason, the data remain eligible in the current scenario even with the availability of such procedures.
In conclusion, our results indicate that patients with small bowel strictures without any mass lesion on imaging and endoscopy findings can have a wide range of diagnoses, including neoplastic disorders. Therefore, we suggest a low threshold for surgical resection in these patients, particularly in older patients with a single stricture in the proximal location. This approach may provide a definitive diagnosis and adequate treatment.
NOTES
AUTHOR CONTRIBUTION: Guarantor of the article; P.S. U.S., acquisition of data, analysis and interpretation of data, drafting of manuscript; S.S., acquisition of data, drafting of manuscript; S.K., interpretation of data, critical revision of manuscript; N.R.D., interpretation of data, critical revision of manuscript; S.P., interpretation of data and critical revision of manuscript; P.D., evaluation of biopsies, drafting of manuscript; V.A., study concept and design, interpretation of data; P.S., study concept and design, interpretation of data, critical revision of manuscript for important intellectual content.
References
1. Kelly ME, McMahon LE, Jaroszewski DE, Yousfi MM, De Petris G, Swain JM. Small-bowel diaphragm disease: seven surgical cases. Arch Surg 2005;140:1162-1166.PMID: 16365236.


2. Wang ML, Miao F, Tang YH, Zhao XS, Zhong J, Yuan F. Special diaphragm-like strictures of small bowel unrelated to non-steroidal anti-inflammatory drugs. World J Gastroenterol 2011;17:3596-3604.PMID: 21987606.



3. Patel S, Gurjar SV. Small intestinal strictures as a complication of mesenteric vessel thrombosis: two case reports. J Med Case Rep 2009;3:8623.


4. Honzawa Y, Kondo M, Hayakumo T, Matsuura M, Nakase H. Successful endoscopic dilation treatment of small intestinal stricture occurring during chemotherapy for malignant lymphoma. Case Rep Gastroenterol 2010;4:323-329.PMID: 21060694.



5. Ahuja V, Tandon RK. Inflammatory bowel disease in the Asia-Pacific area: a comparison with developed countries and regional differences. J Dig Dis 2010;11:134-147.PMID: 20579217.


6. Ahuja V, Tandon RK. Inflammatory bowel disease: the Indian augury. Indian J Gastroenterol 2012;31:294-296.PMID: 23150035.


7. Makharia GK, Srivastava S, Das P, et al. Clinical, endoscopic, and histological differentiations between Crohn’s disease and intestinal tuberculosis. Am J Gastroenterol 2010;105:642-651.PMID: 20087333.



8. Zhou ZY, Luo HS. Differential diagnosis between Crohn’s disease and intestinal tuberculosis in China. Int J Clin Pract 2006;60:212-214.PMID: 16451295.


Fig. 1
Pie chart showing the etiology of small bowel strictures based on the histology of the resected specimens. GIST, gastrointestinal stromal tumor.

Fig. 2
Receiver operating characteristic (ROC) curve for age as a predictor of malignant strictures. Area under the ROC curve, 0.70 (0.55-0.85); sensitivity, 57%, and specificity, 80%.
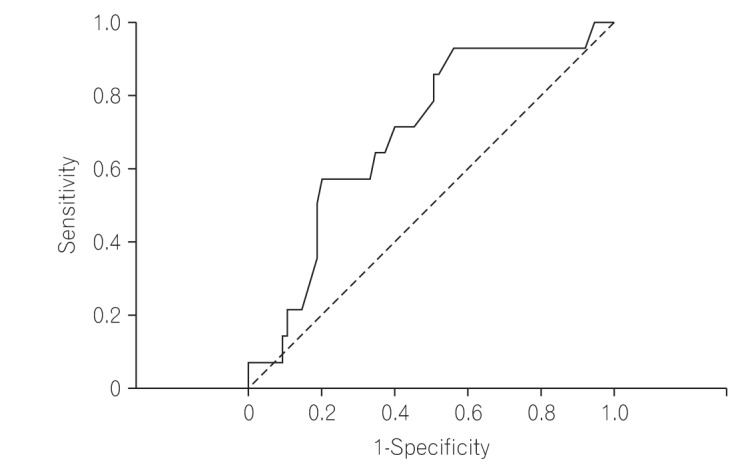
Table 1
Histological Diagnosis According to the Site of Small Bowel Stricture
Table 2
Comparison of Features between Benign and Malignant Strictures
Table 3
Univariate and Multivariate Analysis for the Identification of Factors That Could Differentiate between Intestinal Tuberculosis and CD
- TOOLS






