INTRODUCTION
Non-steroidal anti-inflammatory drugs (NSAIDs) are some of the most commonly prescribed drugs in the world. As a result of their anti-inflammatory, analgesic, and anti-platelet effects, NSAIDs are used in clinical practice for treatment and prevention of rheumatoid arthritis, osteoarthritis, collagen disease, and ischemic cardiovascular or cerebrovascular disease. However, NSAIDs are well known to increase the risk of serious gastroduodenal complications, such as peptic ulcer, bleeding, and perforations.1,2
Until recently, many studies regarding NSAID-induced gastrointestinal (GI) injuries were focused on the stomach and duodenum because of easy accessibility via upper endoscopy. However, recent advances in diagnostic devices used to look at the small intestine, such as capsule endoscopy (CE) and double balloon endoscopy (DBE), enabled direct visualization of the small bowel, and revealed the small bowel injuries induced by NSAIDs.3,4,5,6,7 Graham et al.3 reported that small bowel damage was found in 71% of chronic NSAID users. However, unlike the NSAID-induced gastropathy, the symptoms of NSAID-induced enteropathy are non-specific and the pathogenesis is poorly understood. Additionally, there is no proven effective treatment or prevention for NSAID-induced enteropathy.
Therefore, studies to investigate the mechanism of NSAID-induced enteropathy and treatment modalities for small bowel damage should be performed in the future. In this paper, we intend to describe the current status of small bowel injuries caused by NSAIDs.
EPIDEMIOLOGY
Over the last 10 years, there has been a progressive trend in overall GI complications, such as bleeding and perforation, with a decrease in upper GI complications and an increase in lower GI complications, which include the jejunum, ileum, and large bowel. Thus, the ratio of upper to lower complications has changed from 4.1 in 1996 to 1.4 in 2005.8
Among NSAID users, a 15% prevalence of gastric ulcer and a 10% prevalence of duodenal ulcer was found in an endoscopic study.9 There are several reasons that make it difficult to recognize the prevalence of NSAID-induced enteropathy. First, observing small bowel injuries, induced by NSAIDs, is more difficult than those in the upper GI tract. Second, there is a poor correlation between NSAID-induced small intestinal damage and clinical symptoms. Most symptoms caused by NSAID-induced enteropathy are subclinical and non-specific.10,11 Therefore, until the introduction of CE and DBE, the importance of NSAID-induced enteropathy had been underestimated, compared with NSAID-induced gastropathy.
Recent studies suggested that the damage to the small bowel may occur as frequently and be as severe as upper GI complications.10,11 Allison et al.12 reported the prevalence of small intestinal injuries (ulcerations) in NSAID users and non-users in post mortem patients. Small intestinal ulceration was found in 8.4% of the NSAID users and 0.6% of the non-users. In a study looking at results of capsule enteroscopies, Maiden et al.4 found that small bowel injuries were observed in 68% of healthy volunteers taking diclofenac plus omeprazole for 2 weeks. In another study with 28 rheumatoid arthritis patients, small bowel damage was detected in 13 of 16 patients (81.3%) who used NSAIDs and in 4 of 12 patients (33.3%) who did not.5 In the study using DBE, the NSAID enteropathy occurred in 51% of the patients taking NSAIDs.13 Aspirin seemed to be less harmful to the small bowel in comparison with other NSAIDs.14,15,16 However, even low-dose aspirin can cause bowel damage with short-term administration. Endo et al.14 reported that small bowel injuries occurred more frequently in healthy volunteers to whom low-dose enteric-coated aspirin was administered for 14 days, than in the group not given any drug (80% vs. 20%, P=0.023). Therefore, considering these results, it is necessary to be aware of the adverse effect of NSAIDs not only on the upper GI tract but also the lower GI tract.
PATHOGENESIS
Unlike that of the upper GI tract, the pathogenesis of small bowel injuries caused by NSAIDs was not clearly elucidated in the past because various multiple complicating factors were thought to influence the development of NSAID-induced enteropathy.
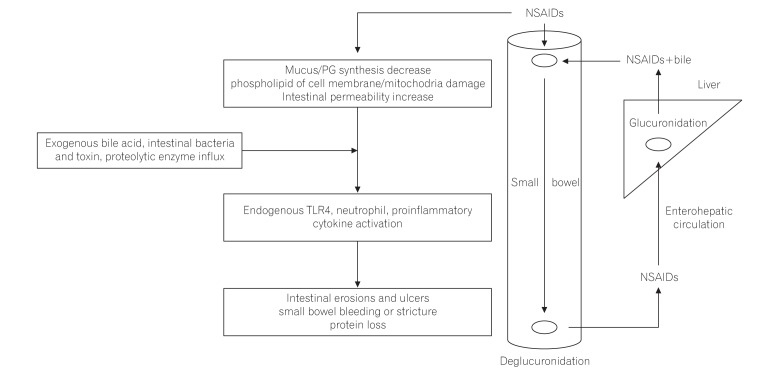
Bjarnason et al.17 suggested a 3-hit hypothesis. First, the phospholipid in the cell membrane on the mucosal surface is directly damaged by NSAIDs and subsequently, injury of the mitochondria in the cell occurs. Second, mitochondrial damage induces the decrease of energy synthesis, resulting in calcium efflux and generation of free radicals. Then, disruption of intercellular junctions and increase of mucosal permeability develop. Third, the intraluminal contents, such as bile acid, proteolytic enzymes, intestinal bacteria, and toxins, can invade the cell through the weakened mucosal barrier, and inflammation develops (Fig. 1).18
PGs have an important role in GI blood flow and mucus production. The depletion of PGs by NSAIDs induces small bowel damage.19,20,21,22 There are 2 type of cyclooxygenase (COX). COX-1 induces PG synthesis and has an important role in maintaining the homeostasis of intestinal mucosa. Previously, only COX-1 inhibition was thought to be associated with mucosal injuries. However, in a recent animal model study, small intestinal damage developed only when both COX-1 and COX-2 were inhibited.23 This indicates that COX-2-derived PGs also play an important role in maintenance of the integrity of the tissue, repairing of mucosal injury, and resolution of inflammation. One study reported that the small bowel injuries by non-selective NSAIDs and selective COX-2 inhibitors were not significantly different in patients on long-term NSAIDs (62% with conventional NSAIDs vs. 50% with selective COX-2 inhibitors, P-value not significant).6
Enterohepatic circulation of NSAIDs also has an important role in the pathogenesis of NSAID-induced enteropathy.24,25,26 The topical adverse effect of aspirin was thought to be localized in the gastroduodenum because of rapid absorption in the stomach and duodenum and the lack of enterohepatic recirculation. To reduce gastric mucosal injuries caused by the topical irritant effect of aspirin, enteric-coated aspirin has been developed.27,28,29,30 Enteric-coated aspirin dissolves mainly in the small bowel, rather than the stomach or duodenum, and enters the enterohepatic circulation, which damages mostly the distal part of the small intestine. When NSAIDs or aspirin did not re-circulate enterohepatically, NSAID-induced small bowel injuries could not develop.31,32 One study reported that small bowel ulcers occurred more frequently in patients taking enteric-coated aspirin (56.3%) than non-coated aspirin (16.7%).14
Intestinal bacteria are important in the pathogenesis of NSAID-induced enteropathy. Germ-free rats and mice had little or no intestinal injuries when given NSAIDs, but when the bowel was colonized by gram-negative bacteria, the small intestine was susceptible to injuries caused by NSAIDs.19,33 In addition, some antibiotics, which work against gram-negative bacteria, were effective in reducing NSAID-induced enteropathy.33,34,35 The lipopolysaccharide of gram-negative bacteria activated the Toll-like receptor 4, which stimulated an inflammatory response, such as cytokine activation and tumor necrosis factor α, triggering nitric oxide (NO) derived from inducible NO synthase and neutrophil activation, resulting in small bowel mucosal injuries.34,35,36,37,38,39 The NSAIDs, acryl glucuronides, which are excreted from the hepatocytes into the bile duct, are deconjugated by an intestinal bacterial enzyme, β-glucuronidase. Subse-quently, NSAIDs could be transported across the epithelium and enter the enterohepatic circulation. The inhibition of β-glucuronidase in an animal model reduced the diclofenac-induced small bowel injuries.40 The low acidity of the gastric environment suppresses bacteria; therefore, chronic acid suppression by proton pump inhibitors (PPIs) can induce bacterial overgrowth and exacerbate the NSAID-induced enteropathy through dysbiosis.41,42,43,44 Similarly, probiotics could decrease the severity of NSAID-induced enteropathy.45
CLINICAL SYMPTOMS AND SIGNS
The signs and symptoms of NSAID-induced enteropathy are usually nonspecific. They have various manifestations, such as iron-deficiency anemia, protein loss, indigestion, constipation, diarrhea, and abdominal pain.10,11 Serious clinical complications, i.e., bleeding, obstruction, and perforation, are infrequent, but can be life threatening.
NSAID-induced enteropathy is associated with occult or overt GI bleeding, resulting in iron-deficiency anemia. In CE, ulcerations and erosions are found commonly in patients taking NSAIDs.4,5,6,7 Kameda et al.46 reported that NSAID-induced enteropathy was the most common etiology of obscure GI bleeding.
Protein losing enteropathy is another clinical manifestation of NSAID-induced enteropathy, which can lead to hypoalbuminemia. Low serum albumin was detected in 10% of rheumatoid arthritis patients taking NSAIDs.47 In a study using 51chromium, the protein loss was observed mainly at the distal ileum level in long-term NSAID users.48
Diaphragm-like stricture is a rare, but pathognomonic, feature of NSAID-induced enteropathy. The numerous concentric, luminal projections of fibrotic submucosal tissue may cause non-specific or obstructive symptoms.49,50 However, because of the intact proper muscle layers, perforation was uncommon. Recently, balloon dilation, instead of surgery, was successfully performed to relieve obstructive symptoms.50 In other studies, NSAID-induced enteropathy was reported to be associated with diverticulitis and diverticular bleeding, vitamin B12 deficiency, and the impairment of bile acid absorption.47,51,52
DIAGNOSIS
The most important reason for underestimating the clinical importance of NSAID-induced enteropathy is the difficulty in making a diagnosis. Before introduction of CE and DBE, the diagnosis of NSAID-induced enteropathy relied on the measurement of small bowel permeability and inflammation. To assay the small bowel permeability, orally administered materials should be shown to be rarely absorbed by an intact small bowel barrier, and absorption should occur only in an area of a damaged intestinal barrier. Then, the absorbed materials are excreted in the urine. Therefore, the severity of small bowel injuries could be confirmed through the amount of the excreted materials in the urine. The permeability of chromium-51-labeled EDTA (51Cr-EDTA) was relatively specific to the small intestine and it was the most widely used in measuring NSAID-induced enteropathy.48,53,54 One study using 51Cr-EDTA permeability showed that the small bowel was more damaged as the dose of NSAIDs was increased (19% with 750 mg naproxen vs. 68% with 1,000 mg naproxen).48 The inflammation of the small intestine could also be detected in scintigraphy using 111indium (In)-labeled leukocytes. The 50% to 70% of patients taking long-term NSAIDs showed enhanced uptake in the small bowel,55 but this test was difficult to use in clinical practice because of its high cost.
Calprotectin, which is a protein in the cytosol of neutrophils, monocytes, and macrophages, can be used as an inflammatory marker of the small intestine. By checking the amount of fecal calprotectin, small bowel injuries caused by NSAIDs could be estimated. One study showed that the level of a single fecal calprotectin was correlated with the 4-day fecal excretion of 111In-labeled leukocytes.53 However, fecal calprotectin had low specificity because it could be elevated in IBD, colon cancer, and other inflammatory conditions.
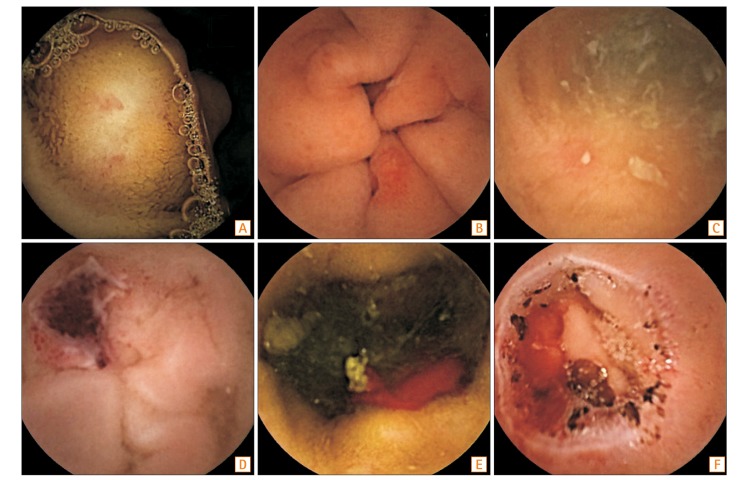
CE and DBE enabled the direct visualization of the small intestine and more exact localization of small bowel injuries. Therefore, the small bowel injuries, caused by NSAIDs, could be better understood using these diagnostic modalities. Compared with DBE, CE is a painless procedure, and has been used easily to evaluate the small bowel injuries caused by NSAIDs. Hayashi et al.56 defined the criteria of NSAID-induced enteropathy as (1) history of NSAID use; (2) endoscopic findings, such as erosions, ulcers, and diaphragm-like strictures; (3) improvement of clinical manifestations and/or endoscopic findings after stopping the NSAIDs; and (4) exclusion of other etiologies, such as IBD, infection, and malignancy. Various findings such as erosions, ulcers, and strictures were found by CE (Fig. 2). Maiden et al.4 divided the NSAID injuries found by CE into 5 categories: petechiae (demarcated areas of crimson mucosa), reddened folds, denuded areas (loss of villi), mucosal breaks (mucosal erosions and/or ulcers), and presence of blood without a visualized lesion.20 In this report, mucosal breaks were detected in about 40% of healthy volunteers after taking 150 mg/day of diclofenac for 2 weeks. In a DBE study, multiple discrete ulcers were found in 28% of patients taking NSAIDs.13 Graham et al.3 reported that mucosal lesions, including red spots, small erosions, large erosions, and ulcers, developed in 13 out of 21 patients (62%) who were chronic NSAID users. As the ability to find small lesions using CE and DBE was not comprehensive, further advanced modalities should be developed.
PREVENTION AND TREATMENTS
The most effective method of preventing NSAID-induced enteropathy is to discontinue the NSAIDs. However, even if temporary withdrawal of NSAIDs is possible, it would be medically contraindicated to stop the NSAIDs continuously in patients with chronic pain or anti-platelet therapy. Therefore, prophylactic drugs are essential in chronic NSAID users, especially if there is suspected small bowel bleeding. Until recently, although many efforts have been attempted, there were no methods or medications to prevent or cure NSAID-induced enteropathy. Medications, such as H2 antagonists, sucralfate, or PPIs, which were developed to minimize upper GI injuries caused by NSAIDs, did not prevent the small intestine injuries.10,11
COX-2 inhibitors produced less gastroduodenal ulceration and bleeding, compared with non-selective NSAIDs. Therefore, COX-2 inhibitors were thought to be less toxigenic to the small bowel.57,58 Goldstein et al.5 showed that small bowel injuries were lower in patients taking celecoxib for 2 weeks, compared with naproxen combined with omeprazole. COX-2 was thought to be associated with maintenance of the integrity of the tissue, repair of mucosal injury, and resolution of inflammation.59,60 However, in patients taking COX-2 inhibitors for more than 3 months, there was no significant difference in the incidence of small bowel injuries between the users of selective COX-2 inhibitors and conventional NSAID users.6,61 Further studies should be performed to determine whether the COX-2 inhibitors can reduce small bowel toxicities.
PG has an important role in mucosal protection; misoprostol, a synthetic PG analog, demonstrated a decrease in the intestinal permeability caused by NSAIDs in several studies.62,63 Watanabe et al.64 reported that misoprostol, but not PPI therapy, was effective in improving mucosal injuries using CE in patients who had developed gastric ulcers when taking low-dose aspirin. Conversely, there was no reduction of the intestinal permeability of 51Cr-EDTA in patients taking indomethacin, even when low dose of misoprostol was given.47 Despite the effectiveness in treating NSAID-induced enteropathy, misoprostol causes many GI adverse effects, such as nausea, indigestion, abdominal pain, and diarrhea.65 These side effects could limit its clinical use.
Rebamipide is a drug that promotes GI mucosal protection by increasing mucus and stimulating PG synthesis.66,67 It also scavenges free radicals and suppresses myeloperoxidase activity. In a study using CE, Niwa et al.68 showed its effectiveness in treating NSAID-induced enteropathy in healthy humans. Small intestine injuries were less frequent in the patients receiving diclofenac for 7 days, together with rebamipide, than in those receiving a placebo (20% with rebamipide vs. 80% with placebo). However, in the larger study, there was no difference between the group taking diclofenac, PPI, and rebamipide for 14 days and the group taking diclofenac, PPI, and a placebo.69 Furthermore, additional studies should be performed to clarify the effectiveness of rebamipide in treating NSAID-induced enteropathy.
Metronidazole is an antibiotic, which is effective against many enteric anaerobic bacteria. Bjarnason et al.70 reported that co-administered metronidazole (with NSAIDs) was effective in reducing NSAID-induced enteropathy. The patients taking NSAIDs received metronidazole and the fecal excretion of 51Cr-labeled erythrocytes and 111In-labeled neutrophils was measured. These 2 fecal inflammatory markers were reduced with the treatment of metronidazole. Recently, several studies have evaluated the potential value of probiotics for prevention or treatment of NSAID-induced enteropathy.45,71,72 In animal models, Lactobacillus acidophilus and Bifidobacterium adolescentis reduced ileal ulcer formation in rats treated with NSAIDs.70 In a clinical trial, CE showed a significant reduction of mucosal breaks in the Lactobacillus casei group compared with the placebo group in patients receiving low-dose enteric-coated aspirin and omeprazole treatment for more than 3 months.45 Additionally, VSL#3, a probiotic formulation consisting of 8 different species of microorganisms, was effective in reducing the fecal calprotectin level in volunteers taking indomethacin.72
PPIs strongly inhibit gastric acid secretion and are prescribed for prevention and treatment of gastroduodenal ulcers by NSAIDs. A previous study found that lansoprazole was effective in reducing small bowel injuries caused by NSAIDs in animal models.73 The mechanism of preventing small bowel injuries by lansoprazole was thought to be by inducing the heme oxygenase-1, which has an important role in inhibiting NSAID-induced small bowel injuries.73,74 Pretreatment with tin-protoporphyrin, which is an inhibitor of heme oxygenase-1, increased the indomethacin-induced enteropathy. On the contrary, lansoprazole exacerbated the small bowel injuries in rats treated with naproxen.75 In a clinical study using CE, small bowel injuries were found more commonly in patients treated with naproxen and omeprazole (55%) than those treated with other medications (16% with celecoxib only, 7% in controls).5 The aggravation of NSAID-induced small bowel injuries by PPIs could be explained by their potential to cause a shift in the types of bacteria in the small intestine (dysbiosis).44 The rats treated with omeprazole had a significant reduction in Actinobacteria, particularly Bifidobacteria, in the jejunum; however, the addition of Bifidobacteria in PPI-treated rats reduced the NSAID-induced intestinal damage.44 For lansoprazole, a clinical trial will be needed to confirm the effect, considering the 2 conflicting reports.
Several drugs, such as NO-releasing NSAIDs and hydrogen sulfide (H2S)-releasing NSAIDs have been developed using the co-drug model. The NO or H2S portions of the co-drugs promote mucosal protection via increasing mucosal blood flow and inhibiting leukocyte adherence to the endothelium. In a clinical trial, NO-naproxen decreased small bowel permeability compared with an equivalent dose of naproxen.75 Additionally, H2S-releasing NSAIDs showed enhanced anti-inflammatory activity in comparison to the conventional NSAIDs.76,77,78
Phospholipid has been proposed to reduce the topical irritant property of NSAIDs.79,80,81 In those studies, it was a component of the epithelial barrier to acid-back diffusion and had an important role in preventing the NSAIDs from disrupting the barrier. The phospholipid lining of the mucosal surface, which was hydrophobic, suppressed the invasion of acid, bile, and other toxic materials. In an animal study, small bowel injuries were not found in the phosphatidylcholine (PC)-indomethacin group.82 In clinical trials, PC-ibuprofen reduced the gastroduodenal injuries, as compared to ibuprofen.83 However, there are no clinical studies regarding the effectiveness of PC-NSAIDs in small bowel injuries.
Sulfasalazine may be a possible treatment modality in NSAID-induced enteropathy. In rheumatoid arthritis patients taking NSAIDs, sulfasalazine reduced intestinal inflammation and blood loss, whereas disease-modifying anti-rheumatic drugs did not.84
CONCLUSIONS
NSAID-induced enteropathy may be as frequent and severe as upper GI complications. In most cases, clinical manifestations are non-specific and pathogenic mechanisms are not well known, but are suspected to be complicated. The new diagnostic modalities, such as CE and DBE, enable diagnosis of small bowel injuries caused by NSAIDs more easily than in the past. However, there is no proven effective medication for treating NSAID-induced enteropathy. Therefore, further studies regarding the prevention and treatment of intestinal injuries caused by NSAIDs are urgently needed.