|
 |
- Search
| Intest Res > Volume 13(3); 2015 > Article |
|
Abstract
With the incidence of inflammatory bowel disease (IBD) increasing rapidly in many Asian countries, including Hong Kong, it is important that patient characteristics are better understood. For example, are the phenotypes, behaviors, complications, and even treatment responses found in Asian patients similar to those of their Western counterparts? To formally address these questions, a properly designed local cohort study is needed. Whilst IBD is still relatively uncommon in Asia, the establishment of a local IBD registry will significantly contribute to the answering of these questions. The Hong Kong IBD registry was established to fill the gap in the understanding of IBD patients, and to foster research into IBD in Hong Kong. The Hong Kong IBD registry is a territory-wide registry that includes all public hospitals in Hong Kong. We included all IBD patients who were currently receiving medical care at these hospitals. With the help of the central computer medical record system of the Hospital Authority of Hong Kong, all clinical events, medications usage, endoscopy records, and laboratory results of patients in the registry were captured. Apart from data collection, the registry is also establishing a bio-specimen bank of blood and stool samples of IBD patients for future research. The IBD registry is a very useful platform for population-based studies on IBD in Asia.
The incidence of IBD has been rising rapidly globally over the past few decades.1 There are wide geographic variations in the incidence and prevalence of IBD, and the disease is still more prevalent in Western countries than in Asia. In Asia, IBD is a rapidly emerging disease, particularly in the more developed countries of the region.2,3 A recent multi-country epidemiological study showed that the incidences of both CD and UC are still significantly lower in most Asian countries than in Australia.4 However, there are wide variations in the incidence of IBD across different Asian countries.
Since most published epidemiological data on IBD originates from Western countries, it remains to be determined whether data published in the West can be directly translated to the Asian setting. In particular, there is a pressing need to validate whether the disease phenotypes, responses to various treatments, and complications are comparable between Asian patients and their Western counterparts.
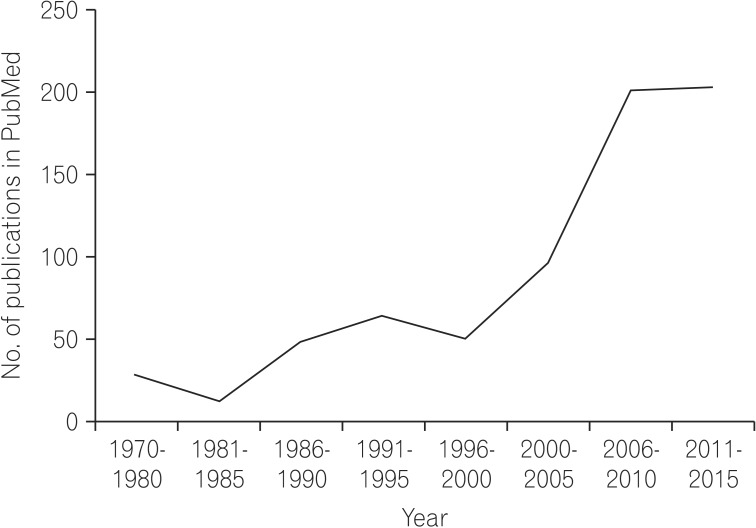
To this end, our Asian colleagues have produced many high-quality publications on the epidemiology of IBD in Asia. A simple search of the PubMed (as in April 2015) database with the keyword "IBD epidemiology in Asia" yields an increasing number of publications over the past few decades (Fig. 1). There were virtually no publications on this topic before the 70's, followed by a surge in the late 90's. However, many of these studies, particularly the early ones, involved small numbers of patients and were based on a few highly selected tertiary referral centers in Asia, which may limit the validity of the results when generalized to the broader region.
With the increasing number of IBD patients in Asia, better design and organization of epidemiological cohort studies are needed. This article attempts to discuss ways of optimizing IBD cohort studies, and to share our early experiences of the recently established Hong Kong IBD registry.
Similar to randomized controlled studies, cohort studies are designed to address clinical questions relevant to disease pathogenesis, characteristics, or management. For IBD, common goals of cohort studies are to characterize epidemiological data on IBD patients, and to collect information on treatment responses and disease related complications and health care utilization. They are also useful in determining other aspects of disease, including various social and psychological parameters.
Prospective cohort studies are capable of obtaining bio-specimens such as colonic biopsy and fecal or blood samples for use in biobanks, which are very useful in subsequent genome-wide association studies as well as microbiota analyses.
Cohort studies are a form of longitudinal study that attempts to analyze target outcomes in a group of individuals. The outcomes can be the occurrence of a target disease among those with exposure to certain risk factors, or the development of a complication among those with a certain disease. In either case, the incidence of disease or the relative risk of exposure can be precisely estimated. In contrast to prospective randomized clinical trials that usually involve intervention, cohort studies are usually observational in nature and patients are not randomly assigned to a specific treatment. This aspect is inferior to randomized controlled trials, as this approach may introduce bias.
The design of these observational studies could be further classified into "prospective" and "retrospective". For instance, a prospective IBD cohort enrolls all eligible IBD patients and follows them longitudinally. All relevant clinical and epidemiological information is recorded at baseline, and updated at intervals during the follow-up period. This may limit recall bias when compared to retrospective cohort studies when the target outcome has already developed. However, some information collected is still retrospectively recalled, particularly in patients with longstanding disease. An inception cohort captures all patients newly diagnosed with IBD only, which minimizes these potential recall biases due to long lag times. This is extremely useful for epidemiological studies designed to determine incidence and exposure. Due to the prospective nature of these studies, they can provide accurate relative risk or risk ratio estimation of target outcomes. Unlike randomized trials that typically assess a single primary outcome, multiple outcomes can be measured. However, inception cohort studies usually require long follow-up periods, and are generally expensive. Problems with patients being lost to follow-up can create uncertainties in data analysis and potential bias.
A retrospective cohort study is conceived after some individuals have already developed the outcomes, e.g., the occurrence of IBD or its related complications. This type of study does not require long follow-up duration and is particularly useful for rare diseases, such as IBD in Asia. These studies are also less costly than prospective cohort studies. Unlike in prospective studies, temporal relationships usually cannot be determined. It is also important to adjust for potential confounding factors in retrospective cohort studies, particularly in selecting cases and determining exposures.
Although intervention is usually lacking for cohort studies, the design and execution, particularly for a prospective cohort study, is very similar to a randomized controlled trial. The effort and resources needed for this kind of study would be comparable to a prospective interventional study. A good example of a long-term cohort study is the Framingham Heart Study, which was started in 1948.5 There are also many examples of long-term IBD cohort studies present in the literature (Table 1).6,7,8,9,10,11,12,13,14,15 Although most of these data are from Western countries, there are a few ongoing Asian cohort studies.4,16
In prospective IBD cohort studies, like inception cohort studies, all patients are identified at baseline and are followed-up longitudinally for target outcomes like the development of complications, mortality, surgical resection etc. Unfortunately, some of these target outcomes may not be identified at the beginning of the study, and are sometimes only considered many years after the study has started. Hence, it is vital to carry out comprehensive and detailed planning for baseline data collection. How often this information needs to be updated is equally important. Since the frequency and extent of follow-up data collection can have major implications on resources and patient compliance, it is prudent to be realistic and practical. Due to the differences in health care systems in different countries, the availability of centralized electronic medical record systems or other similar administrative databases helps to reduce the workload of database maintenance. With long anticipated follow-up durations, mechanisms should be in place to identify patients who have been lost to follow-up due to various reasons.
A team of dedicated IBD researchers is the most important asset of any IBD cohort study. This team should comprise gastroenterologists with a special interest in IBD, research assistants, biostatisticians, technicians, and other related health care personnel. With the many parties involved, regular meetings and progress reports are needed to update all members. A transparent and pre-defined mechanism of sharing the intellectual property obtained from this kind of cohort study, like authorships in publications and the share of patents, is also crucial to maintaining team spirit and avoiding future conflicts. It may also be necessary to establish a publication subcommittee to oversight all research-related publications. The long-term storage of bio-specimens requires proper designated storage space (or freezers) and staff, which can have long-term implications on resource utilization. It is also important to clearly define the rules and regulations governing the future usage of these bio-specimens at the beginning of the study.
As mentioned previously, obtaining adequate funding is another important task for investigators. With the long follow-up duration (sometimes up to a few decades) of cohort studies, the provision of continuous funding support can be a problem for many non-governmental sponsored registries. Hence, a realistic estimate of the sustainability of this cohort study and a frequent revision of the budget may be necessary, particularly for prospective cohort studies.
To ensure compliance with various regulatory authorities, approval from all local institutional review boards concerned should be obtained prior to the implementation of the study. This process can be very time consuming when multiple institutions are involved. Proper informed consent should also be obtained for patients enrolling in prospective studies, and particularly for studies involving the storage of patients' clinical specimens. Special attention should also be directed to maintaining patients' confidentially in the database. Internet security is another important issue that should not be overlooked, particularly with the use of open web-based databases. However, the use of anonymous data may pose problems in subsequent follow-up and data validation.
The Hong Kong IBD Registry was established in 2011 to systematically collect clinical and epidemiological data from IBD patients attending public hospitals in Hong Kong. The registry aims to fill the knowledge gap in the understanding of IBD patients and foster IBD research in Hong Kong. It is a territory-wide registry that includes all public hospitals in Hong Kong. The public hospital system is directly funded by the Hong Kong Government, and is available to all local residents at a minimal cost. It currently provides more than 90% of in-patient services in Hong Kong. The current registry covers 13 public hospitals with a catchment area of more than 7.3 million people. The registry was approved by the local Institutional Review Boards (IRBs) of each individual hospital. Funding was provided by local philanthropists and the pharmaceutical industry, both of which have no role in the daily operations of the registry. A central web-based database was used for data entry from individual participating hospitals. All patients were provided with a unique patient number such that no confidential patient information was stored in the central database.
We prospectively enrolled all patients with a confirmed diagnosis of IBD including CD, UC, and indeterminate colitis. With the help of the central computer medical record system of the Hospital Authority of Hong Kong, which essentially captured all clinical events, prescription histories, endoscopy records, and laboratory results of these patients, important clinical information could be updated periodically. As of April 2014, we collected information from more than 2,200 IBD patients. Results of some of the baseline analyses of these patients were presented in Digestive Disease Week 2015. Apart from data collection, the registry is also setting up a bio-specimen bank of blood and stool samples of IBD patients for future genomic and microbiota studies.
It is an exciting moment for IBD research in the Asia Pacific region, where the disease is emerging rapidly. The formation of IBD study groups and local IBD registries would help to produce valuable epidemiological data on patients, and will ultimately help to transform the care of IBD patients in this region.
ACKNOWLEDGEMENT
I would like to express my gratitude to all investigators who participated in the Hong Kong IBD Registry.
References
1. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142:46-54.PMID: 22001864.


2. Thia KT, Loftus EV Jr, Sandborn WJ, Yang SK. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol 2008;103:3167-3182.PMID: 19086963.


3. Prideaux L, Kamm MA, De Cruz PP, Chan FK, Ng SC. Inflammatory bowel disease in Asia: a systematic review. J Gastroenterol Hepatol 2012;27:1266-1280.PMID: 22497584.


4. Ng SC, Tang W, Ching JY, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn's and colitis epidemiology study. Gastroenterology 2013;145:158-165.PMID: 23583432.


5. Kannel WB, Dawber TR, Kagan A, Revotskie N, Stokes J 3rd. Factors of risk in the development of coronary heart disease-six year follow-up experience. The Framingham Study. Ann Intern Med 1961;55:33-50.PMID: 13751193.


6. Gower-Rousseau C, Vasseur F, Fumery M, et al. Epidemiology of inflammatory bowel diseases: new insights from a French population-based registry (EPIMAD). Dig Liver Dis 2013;45:89-94.PMID: 23107487.


7. Lennard-Jones JE, Shivananda S. EC-IBD Study Group. Clinical uniformity of inflammatory bowel disease a presentation and during the first year of disease in the north and south of Europe. Eur J Gastroenterol Hepatol 1997;9:353-359.PMID: 9160197.


8. Vind I, Riis L, Jess T, et al. Increasing incidences of inflammatory bowel disease and decreasing surgery rates in Copenhagen City and County, 2003-2005: a population-based study from the Danish Crohn colitis database. Am J Gastroenterol 2006;101:1274-1282.PMID: 16771949.


9. Pittet V, Juillerat P, Mottet C, et al. Cohort profile: the Swiss Inflammatory Bowel Disease Cohort Study (SIBDCS). Int J Epidemiol 2009;38:922-931.PMID: 18782896.



10. Beaugerie L, Brousse N, Bouvier AM, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet 2009;374:1617-1625.PMID: 19837455.


11. Ramadas AV, Gunesh S, Thomas GA, Williams GT, Hawthorne AB. Natural history of Crohn's disease in a population-based cohort from Cardiff (1986-2003): a study of changes in medical treatment and surgical resection rates. Gut 2010;59:1200-1206.PMID: 20650924.


12. Martín-de-Carpi J, Rodríguez A, Ramos E, et al. Increasing incidence of pediatric inflammatory bowel disease in Spain (1996-2009): the SPIRIT Registry. Inflamm Bowel Dis 2013;19:73-80.PMID: 22535573.


13. Bernstein CN, Blanchard JF, Rawsthorne P, Wajda A. Epidemiology of Crohn's disease and ulcerative colitis in a central Canadian province: a population-based study. Am J Epidemiol 1999;149:916-924.PMID: 10342800.



14. Hou JK, Kramer JR, Richardson P, Mei M, El-Serag HB. The incidence and prevalence of inflammatory bowel disease among U.S. veterans: a national cohort study. Inflamm Bowel Dis 2013;19:1059-1064.PMID: 23448789.



15. Long MD, Crandall WV, Leibowitz IH, et al. Prevalence and epidemiology of overweight and obesity in children with inflammatory bowel disease. Inflamm Bowel Dis 2011;17:2162-2168.PMID: 21910178.


16. Ishige T, Tomomasa T, Takebayashi T, et al. Inflammatory bowel disease in children: epidemiological analysis of the nationwide IBD registry in Japan. J Gastroenterol 2010;45:911-917.PMID: 20232217.


Table 1
Examples of Population-Based IBD Registries
EPIMAD, registre des maladies inflammatoires du tube digestif du nord-ouest de la France; EC-IBD, European collaborative study group of inflammatory bowel disease; CESAME, cancers et surrisque associé aux maladies inflammatoires intestinales en France; SPIRIT, sociedad española de gastroenterología hepatología nutrición pediátrica; ACCESS, Asia-pacific crohn's and colitis epidemiology study; NA, not available.