Serum albumin is the strongest predictor of anti-tumor necrosis factor nonresponse in inflammatory bowel disease in resource-constrained regions lacking therapeutic drug monitoring
Article information
Abstract
Background/Aims
Evidence on predictors of primary nonresponse (PNR), and secondary loss of response (SLR) to anti-tumor necrosis factor (anti-TNF) agents in inflammatory bowel disease is scarce from Asia. We evaluated clinical/biochemical/molecular markers of PNR/SLR in ulcerative colitis (UC) and Crohn’s disease (CD).
Methods
Inflammatory bowel disease patients treated with anti-TNF agents (January 2005–October 2020) were ambispectively included. Data concerning clinical and biochemical predictors was retrieved from a prospectively maintained database. Immunohistochemistry for expression of oncostatin M (OSM), OSM receptor (OSM-R), and interleukin-7 receptor (IL-7R) were done on pre anti-TNF initiation mucosal biopsies.
Results
One-hundred eighty-six patients (118 CD, 68 UC: mean age, 34.1±13.7 years; median disease duration at anti-TNF initiation, 60 months; interquartile range, 28–100.5 months) were included. PNR was seen in 17% and 26.5% and SLR in 47% and 28% CD and UC patients, respectively. In CD, predictors of PNR were low albumin (P<0.001), postoperative recurrence (P=0.001) and high IL-7R expression (P<0.027) on univariate; and low albumin alone (hazard ratio [HR], 0.09; 95% confidence interval [CI], 0.03–0.28; P<0.001) on multivariate analysis respectively. Low albumin (HR, 0.31; 95% CI, 0.15–0.62; P=0.001) also predicted SLR. In UC, predictors of PNR were low albumin (P<0.001), and high C-reactive protein (P<0.001), OSM (P<0.04) and OSM-R (P=0.07) stromal expression on univariate; and low albumin alone (HR, 0.11; 95% CI, 0.03–0.39; P=0.001) on multivariate analysis respectively.
Conclusions
Low serum albumin at baseline significantly predicted PNR in UC and PNR/SLR in CD patients. Mucosal markers of PNR were high stromal OSM/OSM-R in UC and high IL-7R in CD patients.
INTRODUCTION
Anti-tumor necrosis factor (anti-TNF) agents represent the standard of care for moderate to severe inflammatory bowel disease (IBD). However, approximately one-third of patients receiving anti-TNF therapy develop primary nonresponse (PNR), and a significant proportion (up to 50%) become refractory over time (secondary loss of response, SLR) [1]. Gisbert and Panés [2] estimated that the annual risk for loss of response to infliximab is ~13% per patient-year of treatment.
Hence, predictive biomarkers are warranted which can stratify patients according to their underlying disease biology to improve therapeutic success. Various factors predicting response to anti-TNF have been described, including clinical predictors like disease duration, location, and phenotype; severity of disease; biochemical parameters like hemoglobin, C-reactive protein (CRP), and albumin; treatment-related like previous anti-TNF exposure and combination therapy; and previous surgery [3,4]. Among potential mucosal targets and biomarkers, oncostatin M (OSM), OSM receptor (OSM-R) and interleukin-7 receptor (IL-7R) have gained interest in the past few years [5,6].
With the rising overall burden of IBD in India and other Asian countries, need for anti-TNF agents is also increasing [7]. IBD is as aggressive in India as it is in West, and it must be treated similarly. There is a higher risk of tuberculosis reactivation with anti-TNF agents, however, this risk in endemic areas can be considerably mitigated by using a stringent screening and liberal chemoprophylaxis approach [8,9]. The evidence on predictors of response to anti-TNF agents in these regions is scarce. Moreover, as yet, adalimumab drug levels and antibody tests are not available and access to infliximab drug and antibody levels is very restricted in these regions. Hence in such challenging circumstances managing anti-TNF therapy without access to drug pharmacokinetics necessitates the urgency for evaluating other clinical and biochemical factors which can guide the clinician in planning therapy. Further, the role of therapeutic drug monitoring is limited during the induction phase. Hence, the present study aimed at identifying clinical, biochemical, and molecular markers that predict PNR at week 14 after induction dosing and SLR at week 52 in patients with IBD.
METHODS
1. Patient Population
This study included patients with IBD (ulcerative colitis [UC] and Crohn’s disease [CD]) who received at least induction dosing of anti-TNF therapy and were under follow-up at the IBD Clinic, Department of Gastroenterology, All India Institute of Medical Sciences (AIIMS), New Delhi, from January 2005 till October 2020.
2. Study Design
It was an ambispective analysis of a prospectively maintained database of IBD patients. The following parameters were retrieved from the dataset: demographic features, disease characteristics including location, extent, severity and behavior, presence and number of extraintestinal manifestations (EIMs), age at disease onset and anti-TNF initiation, follow-up duration after anti-TNF initiation, complete blood count, liver and renal functions, history of smoking/alcohol, baseline and follow-up clinical (Simple Clinical Colitis Activity Index [SCCAI] and Crohn’s Disease Activity Index [CDAI]) and endoscopic (Ulcerative Colitis Endoscopic Index of Severity, UCEIS) scores, indication of starting anti-TNF therapy, concomitant immunomodulator use, type of anti-TNF agent and response to therapy. Any missing data was confirmed by interviewing the patient in person. Intestinal mucosal biopsy samples prior to initiation of anti-TNF therapy were retrieved from IBD biorepositories in the department of gastroenterology as well as archived samples stored at the pathology department. Immunohistochemical staining for IL-7R, OSM, and OSM-R was done (Fig. 1). Immunohistochemical staining method has been elaborated in Supplementary Material.
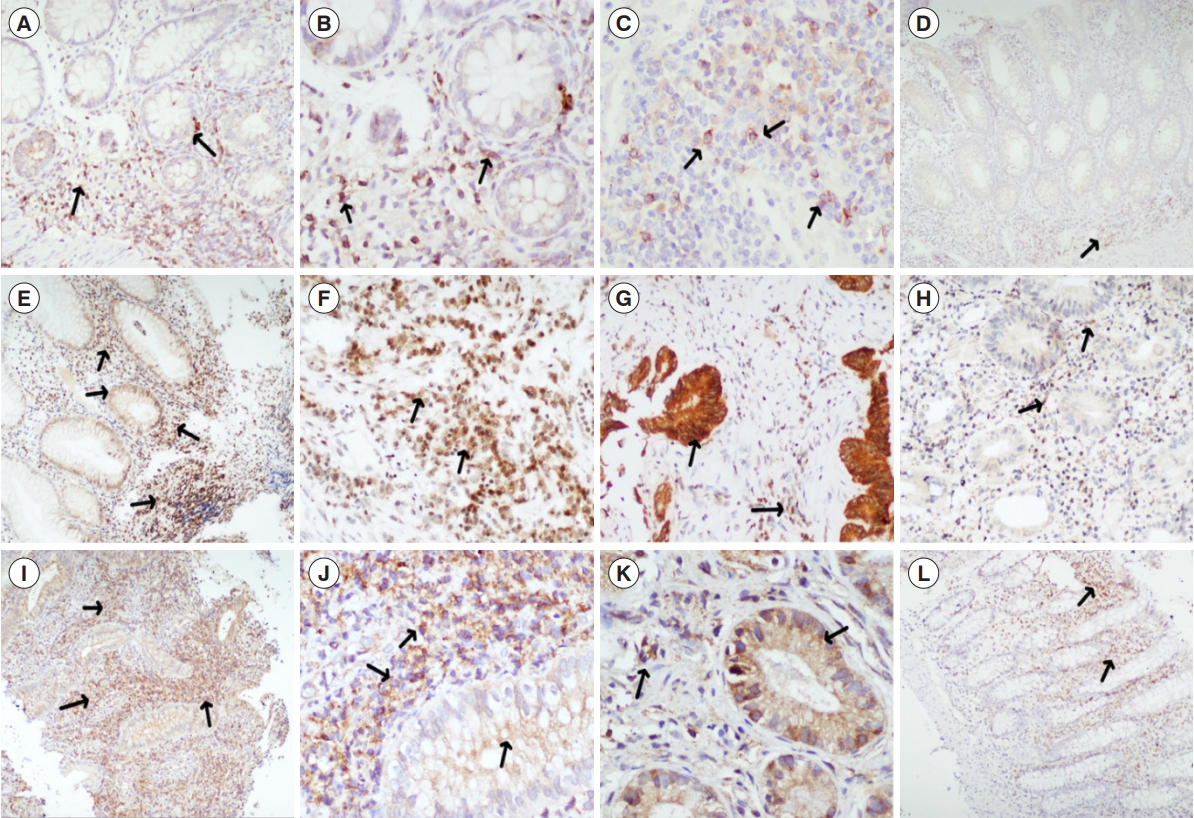
Histopathological finding (immunohistochemical staining). (A-D) Interleukin-7 receptor expression on the stromal lymphocytes and macrophages (arrows) in biopsies from treatment naïve patients with Crohn’s disease, then in disease control, with lesser expression (A: ×100, B: ×200, C: ×200, D: ×40). (E-H) Oncostatin expression in lympho-mononuclear cells, stromal cells as well as in mucosal epithelium (arrows) in colonic biopsies from treatment naïve patients with ulcerative colitis, in comparison to biopsy from disease control, with lesser expression (arrows) (E: ×40, F: ×200, G: ×200, H: ×100). (I-L) Oncostatin-Rß expression in lympho-mononuclear cells, stromal cells as well as in mucosal epithelium (arrows) in colonic biopsies from treatment naïve patients with ulcerative colitis, in comparison to biopsy from disease control, with lesser expression (arrows) (I: ×40, J: ×200, K: ×200; L: ×40).
3. Ethical Considerations
Ethical clearance was obtained from the Institutional Ethics Committee, AIIMS, New Delhi (No: IECPG-599/24.10.19). Additionally, the patients were apprised of the study and their informed consent was obtained.
4. Definitions
Diagnosis of UC and CD was made on the basis of European Crohn’s and Colitis Organisation guidelines [10,11]: (1) Clinical remission was defined as an SCCAI ≤ 2 in UC and CDAI < 150 in CD [12]; (2) Clinical response was defined as decrease in SCCAI score by 3 points in UC and decrease in CDAI by 70 points in CD [12]; (3) PNR was defined as lack of response to induction dose of anti-TNF therapy, assessed at week 14 [13]; (4) SLR was defined as loss of response to anti-TNF therapy after an initial response to induction dose, increase in 3 or more points of SCCAI for 7 consecutive days in patients with UC or an increase in CDAI by more than 70 points from baseline over 2 consecutive weeks in CD patients [14]; (5) Postoperative recurrence was defined as clinical/endoscopic relapse in patients with history of intestinal surgery for CD. Clinical recurrence was defined as CDAI over 150 points or a 70-point increase in CDAI from baseline over 2 consecutive weeks. Endoscopic recurrence was defined as Rutgeerts score of i2 and higher, or evidence of active disease on cross-sectional imaging in patients whom the diseased segment was not accessible by endoscopy; or (6) Early initiation of anti-TNF was defined as therapy started within 2 years of disease diagnosis.
5. Statistical Analysis
Categorical variables were expressed as percentages and continuous variables were expressed mean ± standard deviation or median (interquartile range, IQR) as appropriate. Chi-square or Fisher exact tests were used to compare categorical variables and the Student t-test or Mann-Whitney U test was used to compare continuous variables as appropriate. Univariate and multivariate analysis was done using Cox proportional regression model to predict factors influencing nonresponse (variables with P<0.1 were included in multivariate analysis). P<0.05 was considered statistically significant. Receiver operating characteristic (ROC) curve and area under the curve analysis were done to calculate optimum cutoff of significant predictors of response. Statistical software SPSS version 26 (IBM Corp., Armonk, NY, USA) and STATA 14 (StataCorp., College Station, TX, USA) were used for statistical analysis.
RESULTS
1. Baseline Characteristics
Between January 2005 and October 2020, 6,802 patients with IBD were registered at IBD Clinic, AIIMS, New Delhi. Among them, 186 patients (68 UC and 118 CD) who received antiTNF therapy were included.
2. Ulcerative Colitis
The mean age at disease onset was 28.1 ± 12.3 years, and 58% were males. Mean age at initiation of anti-TNF was 33.4 ± 12.6 years and the median follow-up duration after anti-TNF therapy was 24.5 months (IQR, 14.5–42.0 months). The baseline SCCAI and UCEIS scores were 7.9 ± 0.2 and 4.8 ± 0.1 respectively. Fifty-five patients (80.8%) had pancolitis (E3) and anti-TNF therapy was discontinued in 11 patients due to adverse events.
1) Predictors of PNR
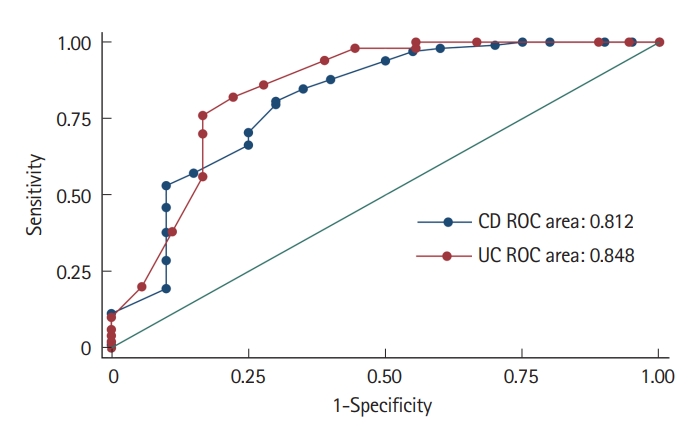
Among 68 patients, 18 (26.5%) had PNR. Mean age at disease onset and age at anti-TNF initiation were numerically lower in patients with PNR, however, it was statistically non-significant. Higher baseline CRP (9.0 mg/L [8.0–12.0] vs. 3.4 mg/L [2.0–6.0], P<0.001) and lower albumin (3.2 ± 0.5 g/dL vs. 3.8 ± 0.3 g/dL, P<0.001) were significantly associated with PNR, other clinical and biochemical parameters were comparable between the 2 groups (Table 1). On ROC analysis, albumin ≤ 3.6 g/dL predicted PNR at week 14 (area under ROC [AUROC] = 0.84) with a sensitivity and specificity of 82.0% and 77.7%, respectively (Fig. 2).

Baseline Demographic, Clinical, Biochemical and Endoscopic Factors Predicting Response in Patients with Ulcerative Colitis at 14 and 52 Weeks (n=68)
2) Predictors of SLR
Thirty-six of 50 patients (72%) who achieved response at 14 weeks maintained it till 52 weeks, and 14 (28%) had SLR over a median duration of 8 months (IQR, 7.2–10 months). There was no significant difference between sustained responders and nonresponders (SLR) in baseline clinical and biochemical parameters (Table 1).
3) Molecular Predictors
Thirty-five patients (51.4%) were included in the analysis of molecular predictors. Twenty-seven patients (77.1%) had response following anti-TNF therapy, whereas 8 (22.9%) had PNR at 14 weeks and 8 of 27 patients (30%) experienced SLR. OSM and OSM-R expression on epithelial cells and IL-7R on stromal cells between responders and nonresponders at 14 (PNR) and 54 (SLR) weeks were comparable. However, higher baseline OSM (7.9 ± 1.5 vs. 5.1 ± 2.8, P=0.02) and OSM-R stromal expression (7.1 ± 1.5 vs. 4.8 ± 2.8, P=0.03) predicted PNR at 14 weeks (Table 2).
3. Crohn’s Disease
The mean age at onset was 28.5 ± 14.0 years and 72 (61%) were males. Mean disease duration at initiation of anti-TNF was 34.5 ± 14.3 years and the median follow-up duration after anti-TNF initiation was 24 months (IQR, 14.2–47.5 months). Median baseline CDAI was 290 (IQR, 260–322) (Table 3). Sixty-two patients (52.5%) had stricturing and 24 (20.3%) had fistulizing phenotype. The perianal fistula was documented in 22 patients (18.6%). Terminal ileal involvement was present in 16 patients (13.5%), isolated colonic disease in 27 (22.8%), ileocolonic location in 34 (28.8%), and 41 patients (34.7%) had proximal small bowel disease. Anti-TNF therapy was discontinued in 16 patients due to adverse events.

Baseline Clinical, Biochemical, and Endoscopic Factors Predicting Response in Patients with CD at 14 and 52 Weeks (n=118)
1) Predictors of PNR
Among the 118 patients, 98 patients had clinical response at 14 weeks and 20 patients had PNR. Lower baseline albumin (2.8 ± 0.5 vs. 3.5 ± 0.4, P<0.01) and postoperative recurrence (25.0% vs. 5.1%, P=0.004) were significantly associated with PNR. Patients with PNR had higher rates of adverse events (40.0% vs. 12.2%, P=0.033) and tuberculosis reactivation rates (20.0% vs. 3.1%, P=0.02) (Table 3). On ROC analysis albumin ≤ 3.3 g/dL predicted PNR at week 14 (AUROC = 0.81) with a sensitivity and specificity of 70.1% and 75%, respectively (Fig. 2).
2) Predictors of SLR
Fifty-two of 98 patients (53.1%) who achieved response at 14 weeks maintained it till 52 weeks, and 46 (46.9%) had SLR over a median duration of 7 months (IQR, 5–9 months). Among the clinical predictors, presence of EIM (58.6% vs. 34.6%, P=0.02) was significantly associated with SLR. Lower baseline albumin was also significantly associated with SLR (3.3 ± 0.4 g/dL vs. 3.7 ± 0.4 g/dL, P<0.001). On ROC analysis, albumin ≤ 3.6 g/dL (AUROC = 0.70) predicted SLR at week 54, with a sensitivity and specificity of 62.7% and 56.5%, respectively (Supplementary Fig. 1). Other biochemical parameters were comparable between the 2 groups (Table 3).
3) Molecular Predictors
Twenty-eight pretreatment biopsy samples were analyzed for molecular predictors of response. Five of 28 patients (17.8%) experienced PNR. Of the 23, 8 patients (34.7%) experienced SLR. OSM and OSM-R expression on stromal and epithelial cells between responders and nonresponders at 14 and 54 weeks were comparable. However, higher baseline IL-7R expression on stromal cells predicted PNR at 14 weeks (6.8 ± 2.2 vs. 3.5 ± 2.7, P=0.02) (Table 2).
4. Univariate and Multivariate Analysis
1) Ulcerative Colitis
On multivariate analysis only low serum albumin (hazard ratio [HR], 0.11; 95% confidence interval [CI], 0.03–0.39; P=0.001) predicted PNR. Due to small sample size, molecular predictors (OSM stromal and OSM-R stromal) could not be accounted for in multivariate analysis (Table 4).
2) Crohn’s Disease
On multivariate analysis only low serum albumin (HR, 0.09; 95% CI, 0.03–0.28; P<0.001) predicted PNR. Due to small sample size, molecular predictors (IL-7R) could not be accounted for in multivariate analysis (Table 4). On multivariate analysis, only low baseline albumin predicted SLR (HR, 0.31; 95% CI, 0.15–0.62; P=0.001) (Supplementary Table 1).
DISCUSSION
This study has evaluated clinical and molecular predictors of response to anti-TNF therapy in Asian patients with IBD. Predicting anti-TNF therapy success in areas without access to drug pharmacokinetics as well as molecular predictors have not been described for any Asian cohort as yet and this study fulfils that gap. Low serum albumin prior to anti-TNF therapy initiation significantly predicted PNR in UC patients and PNR as well as SLR in CD patients. Mucosal markers of PNR were high stromal OSM and OSM-R expression in UC and high stromal IL-7R expression in CD patients.
This study highlights foremost that only 2.7% of patients (186/6,802) had access to anti-TNF therapy highlighting the resource gap in this region. Phenotypic descriptive studies have shown that IBD in India is behaviorally similar to IBD patients from the West [8], yet the dismal rate of usage of anti-TNF therapy suggests lack of access to high-cost medicines as universal health insurance coverage is absent. And in line with that is the absence of adalimumab drug and antibody level measurement facilities and restrictive access to drug pharmacokinetic measurements of infliximab. Hence, the research gap in such regions is to evaluate if easily measurable clinical and biochemical factors can be helpful in such situations and wherein lies the significance of this study.
Amongst the clinical and biochemical factors, only higher baseline CRP and lower albumin were significantly associated with PNR in UC (on univariate analysis), however, only albumin retained significance on multivariate analysis. Patients with active inflammation, characterized by elevated levels of inflammatory biomarkers such as CRP are less responsive to anti-inflammatory therapy as seen in previous studies [3,15], where a high baseline CRP (> 5 mg/L) was an independent predictor of colectomy in UC patients using infliximab [15]. High CRP reflects a higher inflammatory load, contributing to faster drug elimination and reduced drug response. Higher baseline CRP levels have also been associated with nonresponse in patients with CD, as suggested in a retrospective cohort of 148 CD patients, where high baseline CRP levels predicted PNR [16]. However, higher CRP levels could also select patients with active disease who are likely to respond to anti-TNF therapy as documented in a real-life cohort of 438 CD patients, where an elevated CRP was associated with a 3-fold higher response rate to induction therapy with adalimumab [17]. However, no such significant association between baseline CRP levels and nonresponse (or response) was seen in our cohort of patients with CD. CRP levels are determined by multitude of factors such as genetic polymorphisms, ethnicity, and sociodemographic and environmental variables, body mass index being one of the most important correlates accounting for 15% variation in CRP [18]. As per World Health Organization Global Health Observatory 2016 estimates, body mass index of Indian adults is lower than Western counterparts, which could account for low CRP in Indian population, and hence lack of association of CRP with TNF response. A recently published multicenter study on acute severe UC demonstrated similar observations with CRP in the Indians being lowest as compared to British and Australians [19].
Albumin plays a cardinal role in drug transport and metabolism by binding several drugs with a high affinity, thereby slowing their elimination rates [20]. Low albumin concentration is a marker of underlying pathologic processes, such as malnutrition and inflammation, and has been linked to poor outcomes in many health disorders [21]. Here, we emphasize the significance of serum albumin as a biomarker in predicting nonresponse to anti-TNF therapy. Anti-TNF titers are negatively influenced by low serum albumin levels, as demonstrated in a study of 720 patients by Fasanmade et al. [22], where higher serum albumin maintained a higher infliximab concentration, lower clearance, and longer half-life than patients with lower serum albumin. In the present study, PNR was documented in both UC and CD patients with low baseline serum albumin. Moreover, lower baseline albumin was also associated with SLR in CD patients. Like our study, several studies have correlated serum albumin with anti-TNF drug levels and responses in patients with both UC and CD. In a recent study, patients with acute severe UC had significantly lower infliximab levels compared to those with moderate UC during the induction phase, which significantly correlated with albumin levels [23]. In another study of 285 patients with steroid-refractory UC treated with infliximab, baseline albumin ≥ 3.5 g/dL was an independent predictor of colectomy-free survival [3]. Similarly, in a study of 64 UC patients, and in the PANTS study (UK-wide, multicenter, prospective observational cohort study in CD), low baseline serum albumin predicted anti-TNF nonresponse (P<0.05) [13,24]. The relationship between serum albumin and anti-TNF response, mechanistically can be explained by the common mechanism which catabolizes both albumin and monoclonal antibodies (which are IgG immunoglobulins), namely the neonatal Fc receptor [22]. These findings suggest that improving albumin levels can increase the anti-TNF response, and same has been corroborated by improved anti-TNF response in combination with exclusive enteral nutrition [25].
Among other clinical predictors, postoperative recurrence (PNR), EIMs (SLR) and adverse events (PNR) predicted nonresponse in CD on univariate analysis. Lack of association between adverse events and response in UC could be due to low sample size. Postoperative recurrence indicates an aggressive disease phenotype, and thus poor response to anti-TNF, similar findings were demonstrated in a Belgian study [3]. Similarly, presence of EIMs would also indicate higher inflammatory burden leading to loss of response. Adverse events would require intermittent or permanent withdrawal of anti-TNF therapy thereby leading to higher nonresponse. Other factors which demonstrated a trend but were not significantly associated included lower age at disease onset, smoking, and concomitant steroid use (for PNR); and presence of EIM and late age at anti-TNF initiation (SLR) in UC, and past history of antitubercular treatment (both PNR and SLR), higher baseline CDAI (PNR), perianal disease (SLR) and absence of smoking in CD (SLR) (Tables 1 and 3). Most of these factors have been heterogeneously associated in other studies and either indicate higher inflammatory burden at anti-TNF initiation or an aggressive disease course [26]. A reverse association between smoking and loss of response with anti-TNF therapy could be due the lack of association between smoking and disease course in CD as demonstrated in earlier studies from India [27]. Anti-tubercular therapy has been associated with aggressive disease course in CD [28], and the same factor could account for the trend observed in present study. Low sample size could account for lack of significance.
OSM, a member of the IL-6 family of cytokines which shares common gp130 receptor subunit, is one of the most strongly and consistently expressed cytokine in the colon of IBD patients when compared to healthy colonic mucosa [5], and enhances intestinal inflammation by upregulating the expression of chemokines, cytokines and adhesion molecules on gut-resident stromal cells. In a landmark study, analysis of more than 200 patients with IBD demonstrated that a high pretreatment OSM level was strongly associated with failure of anti-TNF therapy [5]. Similar results were seen in our study, where higher baseline OSM stromal levels predicted PNR at 14 weeks in patients with UC. Due to the small sample size of patients with molecular markers, OSM and OSM-R could not be included in multivariate analysis.
IL-7 regulates T lymphocyte homeostasis and is primarily produced by epithelial and stromal cells. IL-7R regulates the expression of both pro and anti-apoptotic BCL-2 family members and activates the PI3K and JAK/STAT pathways to provide proliferative and antiapoptotic signals [29]. A recent study demonstrated heightened expression of the IL-7R and the IL-7 dependent signaling pathway in the inflamed colon of IBD (120 UC and 8 CD) patients nonresponsive to anti-TNF therapy [6]. In the present study also higher baseline IL-7R expression on stromal cells in CD patients predicted PNR at 14 weeks. In UC, although expression of IL-7R was numerically higher in patients with PNR, the difference was statistically non-significant.
The use of anti-TNF is increasing in Asia with the rising disease burden of IBD. However, because of their prohibitive cost and increased chances of adverse events, it is important to develop the predictors of response to anti-TNF in patients with IBD from India and similar countries. This study is the first such attempt from Asia and lays a platform for further larger studies toward this goal. Limitations include retrospective design and relatively small sample size, and therefore our results require further validation in larger cohorts. More objective endpoints, such as mucosal healing on endoscopy and transmural healing on cross-sectional imaging, were not investigated; instead, clinical endpoints were employed to identify nonresponse. Molecular markers–OSM, OSM-R, and IL-7R were examined in relatively few biopsy specimens; hence, these variables could not be included in multivariate analysis. AntiTNF levels and antibody levels are crucial indicators of nonresponse, but we were unable to evaluate these indicators.
In conclusion, our findings suggest low serum albumin as the most important predictor of nonresponse to anti-TNF therapy in patients with IBD when drug pharmacokinetic measurement is unavailable. Molecular predictors such as stromal OSM, OSM-R, and IL-7R pathways provided a signal, and will require further validation in studies with larger sample sizes.
Notes
Funding Source
The research was funded by the Indian Council for Medical Research - Centre for Advanced Research and Excellence in Intestinal Diseases under grant number 55/4/11/CARE-ID/2018-NCD.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Data Availability Statement
Not applicable.
Author Contribution
Conceptualization: Ahuja V. Data curation: Kumar P, Vuyyuru SK, Das P, Kante B, Ranjan MK, Mundhra S, Sahu P, Mouli VP, Golla R, Kedia S. Formal analysis: Kumar P, Vuyyuru SK, Das P, Kedia S. Funding acquisition: Kedia S, Ahuja V. Investigation: Kumar P, Vuyyuru SK, Kante B, Ranjan MK, Mundhra S, Ahuja V. Methodology: Kumar P, Vuyyuru SK, Das P, Kante B, Sahu P, Mouli VP, Singh MK, Ahuja V. Project administration: Ahuja V. Resources: Kumar P, Das P, Kedia S, Ahuja V. Software: Ahuja V, Kedia S. Supervision: Kumar P, Vuyyuru SK, Das P, Sharma R, Kedia S, Ahuja V. Validation: Kumar P, Sharma R. Visualization: Ahuja V. Writing - original draft: Kumar P. Writing - review & editing: Kumar P, Vuyyuru SK, Das P, Kante B, Ranjan MK, Thomas DM, Mundhra S, Sahu P, Mouli VP, Jain S, Goyal S, Golla R, Virmani S, Singh MK, Sachdeva K, Sharma R, Dash NR, Makharia G, Kedia S, Ahuja V. Approval of final manuscript: all authors.
Supplementary Material
Supplementary materials are available at the Intestinal Research website (https://www.irjournal.org).
Supplementary Material.
ir-2022-00128-Supplementary-1.pdfSupplementary Fig. 1. Receiver operating characteristic (ROC) curves for baseline albumin levels as predictor of nonresponse to anti-tumor necrosis factor therapy at week 52 in Crohn’s disease patients.
ir-2022-00128-Supplementary-Fig-1.pdfSupplementary Table 1. Variables Associated with Secondary Loss of Response to Anti-Tumor Necrosis Factor Therapy among Patients with Crohn’s Disease
ir-2022-00128-Supplementary-Table-1.pdf