|
 |
- Search
| Intest Res > Volume 16(4); 2018 > Article |
|
Abstract
Background/Aims
Testing for hepatitis B virus (HBV) serologic markers and appropriate vaccination are required in the management of inflammatory bowel disease (IBD) patients. We evaluated immunogenicity for HBV in IBD patients and the response to the HBV vaccination.
Methods
From May 2014 to August 2016, patients diagnosed with IBD were prospectively included and evaluated for antibody to hepatitis B surface antigen, antibody to hepatitis B core antigen, and antibody to hepatitis B surface antigen. Among the 73 patients who were confirmed with nonimmunity to HBV, 44 patients who had completed the 3-dose HBV vaccination series received a single booster vaccination, while 29 patients who had not completed the vaccinations series or were unsure of receiving the vaccination received a full vaccination series.
Results
An optimal response was obtained in 70.5% of the patients in the booster group, and 89.7% of the patients in the full vaccination group. Age younger than 26 years (odds ratio [OR], 6.01; 95% confidence interval [CI], 1.15-31.32; P=0.033) and a complete previous vaccination series (OR, 0.15; 95% CI, 0.03-0.80; P=0.026) were associated with optimal vaccine response. Previous complete vaccination series (OR, 0.11; 95% CI, 0.02-0.73; P=0.022) was the only predictive factor for lower compliance.
Conclusions
The response to the HBV vaccination was lower in patients older than 26 years and for those patients with a complete vaccination history. Since patients with a complete vaccination history also had poor compliance, serum HBV-titers should be checked more thoroughly, and a full vaccination series should be administered in cases when there is a negative response to the booster vaccination.
Patients with IBD are at increased risk of infectious disease raised from the nature of disease, immunosuppressive agents such as biologics and immunomodulators, and procedures including endoscopy, transfusion, and surgery, which are frequently required during treatment [1,2]. Infectious diseases often lead to higher morbidities and mortalities in IBD patients who are generally in an immune-suppressed status. Therefore, effective vaccinations against these diseases should be administered.
HBV infection has been reported to cause fulminant or fatal hepatitis by opportunistic infection or reactivation of HBV in IBD patients, but is preventable with a vaccine [3-6]. The American College of Gastroenterology (ACG) clinical guidelines recommend HBV vaccination for every IBD patient lacking HBV immunity with non-immunization regardless of immunosuppression status, especially before the start of anti-tumour necrosis factor (anti-TNF) therapy [7]. Since anti-HBs levels decline over time [8], ACG guidelines also recommend a regular check of anti-HBs titer to confirm immunity [7]. A booster vaccination or a new vaccination series is recommended for patients with insufficient protective antibody concentrations [7].
As a part of the National Immunization Program, all neonates have received a universal HBV vaccine since 1995 in Korea, regardless of maternal HBsAg status [9]. With the implementation of the national vaccination program, HBsAg prevalence has decreased from 10% in the 1980s [10] to 2.9% in 2013 [11]. However, it has been reported that the decline of HBV infection is limited to the younger population whereas the prevalence of HBV infection in the middle-aged and elderly populations has remained unchanged [9]. HBV infection is still the leading cause of chronic liver disease, including chronic hepatitis, cirrhosis, and hepatocellular carcinoma [12]. Therefore, it is important to monitor the status of HBV immunity and vaccinate IBD patients who lack HBV immunity.
In this study, we aimed to assess the following: (1) the HBV immune status in patients with IBD; (2) the response to a booster HBV vaccination in patients who had previous completed a 3-dose vaccination series or the response to a 3-dose HBV vaccination series in patients with incomplete or an unknown vaccination history; (3) the predictive factors for an effective HBV vaccine response; (4) the response to a new complete vaccination series (revaccination) in patients who had shown no response to a booster vaccination; and (5) the factors associated with compliance to the recommended vaccination schedule.
Patients from the IBD clinic in Ewha Womans University Mokdong Hospital who provided informed consent were enrolled prospectively from May 2014 to August 2016. After informed consent was obtained, peripheral blood samples were collected to evaluate HBV serologic markers (anti-HBs, HBsAg, and anti-HBc).
Patients who were diagnosed with IBD by clinical, endoscopic, radiographic, and histological assessment and older than 15 years at the time of screening were included. Subjects with severe comorbidities including liver cirrhosis, chronic renal failure, and malignant disease were excluded. For the analysis of vaccine response and response associated factors, only the patients who were confirmed with negative serologic results for anti-HBc, and anti-HBs, and negative serologic result for HBsAg were included.
Prior HBV infection included resolved HBV infection or remote infection, and each category was defined by the presence of anti-HBc and anti-HBs or the presence of anti-HBc without anti-HBs, respectively. Present HBV infection, which included carrying an inactive HBsAg, and chronic hepatitis B were defined as the presence of HBsAg. Immunity after prior vaccination was defined as the presence of anti-HBs without anti-HBc [2,13,14]. The Institutional Review Board of Ewha Womans University Hospital approved this study (IRB No. 2014-06-026-008).
The vaccine used in this study was the Hepavax-Gene TF® (purified hepatitis B type surface antigen, 1.0 mL, 20 μg) manufactured by Green Cross Corp (Yongin, Korea). The vaccine was injected into the deltoid muscle.
Among the patients who were confirmed with nonimmunity to HBV, patients who had been previously vaccinated with a 3-dose HBV vaccination series received a booster vaccination (booster group), and the patients who had not completed the hepatitis B vaccination series or were unsure of their vaccination history were administered a 3-dose HBV vaccination in months 0, 1, and 6 (full series vaccination group). A new complete 3-dose vaccination was administered to patients who did not acquire adequate immune response with a booster vaccination. All patients were instructed on possible adverse events related to the HBV vaccination and to report their symptoms to the study staff as soon as possible.
Good compliance was defined as patients who had received every recommended vaccination. Only those patients who had shown good compliance were included in the evaluation of vaccine response.
The serum anti-HBs level was measured at least 1 month after either the booster vaccination or the last of the 3-dose vaccination series. The response was considered as optimal if the anti-Hbs titer was equal to or higher than 10 IU/L. These responses were sub-categorized using different anti-HBs cutoff points: effective immune response (EIR, anti-Hbs ≥100 IU/L) and adequate immune response (AIR, anti-Hbs ≥ 10 IU/L). A serum anti-HBs level below 10 IU/L was considered as non-responsive [1].
The following demographic and clinical data were collected to analyze predictive factors associated with vaccine response and compliance: demographic information (age at the first dose vaccination in this study and sex), comorbidities, IBD subtype, disease duration (interval between IBD diagnosis and vaccination), disease severity, treatment at the time of first dose vaccination in this study, and the indication for vaccination. Disease severity was assessed using a partial Mayo score (if <5, mild; if 5-7, moderate; if >7, severe) for UC [15] and a CDAI (if 150-219, mild; if 220-450, moderate; if >450, severe) [16] for CD. The types of treatment were categorized into 3 group: (1) biologics group, which includes infliximab, adalimumab, golimumab, or any combination with biologics; (2) an immunosuppressive group, which includes corticosteroids, azathioprine, methotrexate, cyclosporine A, or any combination of these drugs with aminosalicylates; and (3) a non-immunosuppressive group (only aminosalicylate).
All statistical analyses were carried out using the SPSS program, version 22.0 (IBM Corp., Armonk, NY, USA). Continuous variables are presented as the mean with standard deviation, and categorical variables are presented as number with percentage. In univariate analyses, Student t-test was used for continuous variables, and the chi-square test, Fisher exact test, or linear-by-linear association was used for categorical variables. Multivariate logistic regression analysis was used to identify independent predictive factors for optimal vaccine response and compliance. P-values <0.05 were considered statistically significant.
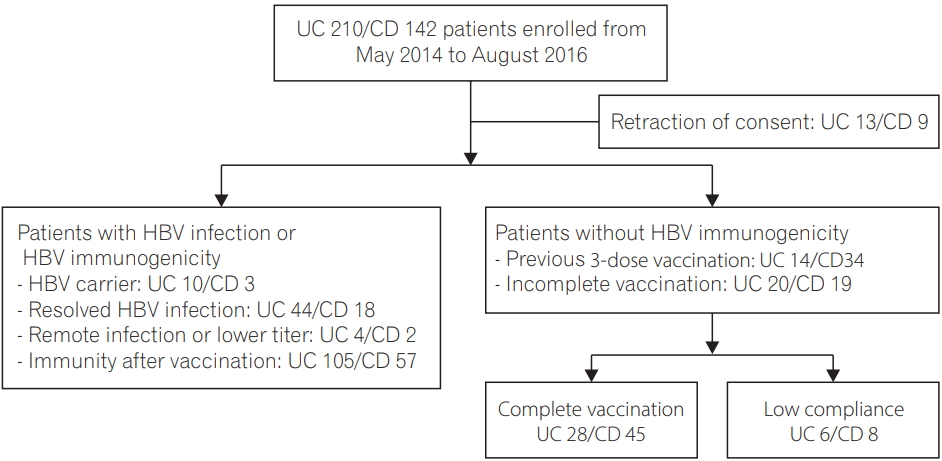
A total of 352 patients with IBD (210 UC, 142 CD) were enrolled in this study. After excluding 22 patients who retracted the consent, (UC 13, CD 9), a total of 330 patients were included in this study (Fig. 1). The mean age of the patients was 38.5 years, and 62.7% of patients were male. One hundred and ninety-seven patients (59.7%) were diagnosed with UC, and 133 (40.3%) were diagnosed with CD. Regarding the HBV immune status, 81 patients (24.5%) had prior or present HBV infection (13 HBV carrier [UC 10, CD 3], 62 resolved HBV infection [UC 44, CD 18], 6 remote HBV infection [UC 4, CD 2]), 162 patients (49.1%) had immunity after prior vaccination (UC 105, CD 57), and 87 patients (26.4%) did not have immunity to HBV (UC 34, CD 53).
Among 87 patients who had nonimmunity to HBV and were decided to receive HBV vaccination, 14 patients who showed low compliance to vaccination were excluded for further analysis. Baseline characteristics of vaccinated patients are shown in Table 1. The mean age at the time of initial vaccination in this study was 29.9 years, and 67.1% of patients were male. Sixty patients (82.2%) had maintained disease activity from remission to mild status under the prescribed medication. Regarding the indication for HBV vaccination, 44 patients with a previous complete HBV vaccination (60.3%; UC 13, CD 31) received a booster vaccination, while 29 patients without prior vaccination or an unknown history of vaccination (39.7%; UC 15, CD 14) received a full 3-dose vaccination. There were no severe adverse events related to vaccination during the study.
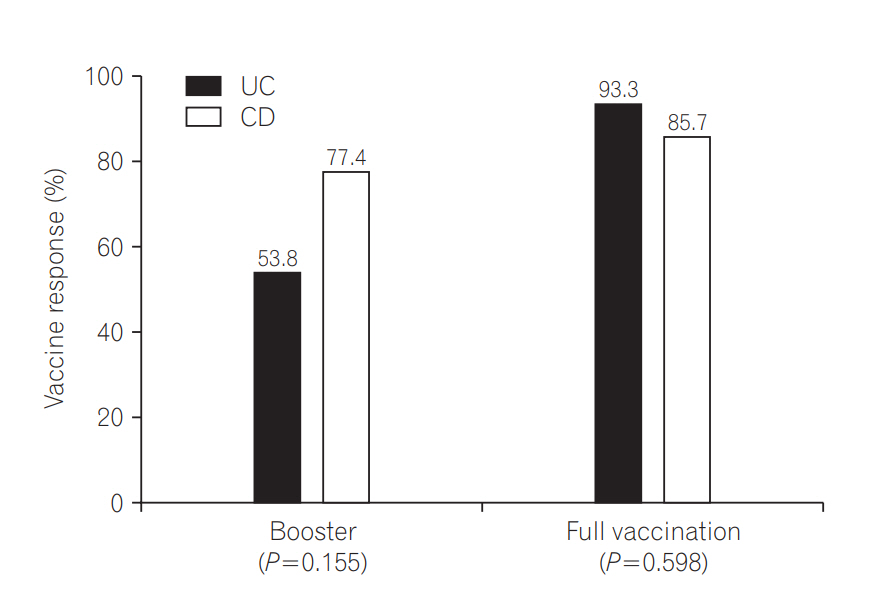
Among the 44 patients who received a booster vaccination, 31 (70.5%) obtained optimal responses. There were no significant differences between optimal vaccine response and disease type (53.8% UC and 77.4% CD, respectively, P=0.155). Among the 29 patients who received the full HBV vaccination series, 26 (89.7%) showed optimal responses. There was also no significant difference between optimal vaccine response and disease type (93.3% UC and 85.7% CD, respectively, P=0.598) (Fig. 2).
When vaccine responses were analysed, the EIR and AIR of UC patients were slightly lower than in CD patients in the booster vaccination group, whereas the EIR and AIR of UC patients were higher than in CD patients in the full vaccination group. However, these differences were not statistically significant (Fig. 3).
To identify predictive factors for vaccine response, the associations between baseline clinical characteristics of enrolled patients and optimal vaccine response were analyzed. In univariate analysis, there were no statistically significant predictive factors. However, in a multivariate analysis that included variables that were considered as clinically significant, a younger age at the initial vaccination dose (<26 years) was a positive predictor for optimal vaccine response (OR, 6.01; 95% CI, 1.15-31.32; P =0.033). In addition, a previous complete vaccination history (OR, 0.15; 95% CI, 0.03-0.80; P =0.026) was a negative predictive factor for optimal vaccine response (Table 2).
Among the 44 patients who received a booster vaccination, a total of 13 (UC 6, CD 7) failed to achieve optimal vaccine response. These patients were recommended to receive 2 more dose vaccinations. The mean age of this population was 28.3 years, and 8 patients (61.5%) were male. Eleven patients (84.6%) had remission to mild disease severity and were taking the following categories of medications: 2, biologics monotherapy or combination therapy; 7, immunomodulators; and 4, non-immunomodulators. Optimal vaccine response was observed in 10 patients (76.9%, UC 83.3%; CD 71.4%) (Fig. 4).
To identify predictive factors for compliance with the recommended vaccine schedule, the clinical characteristics of the 73 patients with good compliance and the 14 patients with poor compliance were compared. A complete previous vaccination history was the only significant factor associated with lower compliance in both univariate analysis (P=0.029) and multivariate analysis (OR, 0.11; 95% CI, 0.02-0.73; P=0.022) (Table 3).
This study is the first to prospectively analyze HBV vaccine response in patients with IBD in Korea, an intermediate endemic area for HBV. We found that 26% of IBD patients did not have immunity to HBV, 49% of patients had maintained immunogenicity against HBV from a prior vaccination, and 25% of patients had prior or present HBV infection. In regard to the vaccination response, 70.5% of patients obtained an optimal response from the booster vaccination, and 89.7% of patients acquired an optimal response from the full vaccination series.
HBV vaccination is an effective method for reducing infection and related serious complications, including cirrhosis or hepatocellular carcinoma. In Korea, HBV vaccination prevalence has exceeded 99% since 1990 [17], which has resulted in dramatic decreases of HBsAg seropositive rates (2.9% in the general population) [11] and HBcAb seropositive rates (10.6% in adults younger than 30 years) [17]. The non-immunity rate for HBV in the general population is reported as 19.7% [18].
Compared to the general population in Korea, our study showed that IBD patients have higher risk of HBV infection and suboptimal response to HBV vaccination. Among the patients who did not have immunity, only 55% had a history of the complete vaccination series, whereas the other patients had not completed the vaccination series or the vaccination history was unknown. Recent Korean studies [19] reported an HBV vaccination rate of 52.6% in IBD patients, which is higher than other vaccination rate, such as measles-mumps-rubella (42.2%), influenza (37.5%), varicella (34.9%), and hepatitis A (15.6%). However, this rate still falls short. The primary reason given for not receiving a vaccination was “not knowing the existence or necessity of vaccine.” The low rate of HBV vaccination in IBD patients is worrisome because an endogenous immunosuppressive state arising from altered immunity and an exogenous immunocompromised state due to immunosuppressive drugs can increase the risk of opportunistic infections [19].
Generally among healthy individuals, a 3-dose vaccination induces a 90% to 95% protective antibody concentration [20]. Although antibody concentration wanes over time after the primary immunization, the protection can persist for at least two decades [21]. Moreover, protection against disease could persist even after the disappearance of anti-HBs antibodies in individuals with an intact immune system [22]. Therefore, further vaccination is usually not recommended in individuals with full response to the complete vaccination series [21].
However, IBD patients have shown diminished vaccine response in the range of 33% to 76% [23] due to aberrant Th1/Th2 immune response, which led to disturbance of cytokine secretion and ineffective vaccine response, as well as concurrent use of immunosuppressive therapy [18]. In addition, low initial antibody level was also correlated with decreased antibody persistence [24]. Thus, 2017 ACG clinical guidelines recommend testing for HBV serologic markers and to vaccinate non-immune patients, particular before anti-TNF treatment [7]. For patients with suboptimal antibody concentration, a single dose booster vaccine should be considered, and if the response is not optimal, a full vaccination series is recommended [7]. Booster vaccinations are known to increase antibody levels rapidly by triggering memory B cells, which have been sensitized by the primary vaccine to proliferate and differentiate [22].
Based on this guideline, we evaluated the response of HBV vaccination in 2 groups classified by prior complete vaccine experience, namely the booster group and the full vaccination group. As expected, the response rates in both groups was lower than the levels within the general population, 70.5% versus 88% [24] for booster group and, 89.7% versus 90% to 95% [20] for full vaccination group, respectively. Young age was a significantly positive predictive factor for optimal vaccine response, whereas prior complete vaccination was a significantly negative factor. Disease type, duration, activity, and type of treatment at the time of vaccination were not significant factors.
Age has been known to have an inverse correlation with vaccine response [25-27]. Immunosenescence, an aging process of the immune system including thymic involution, changes in cytokine production/distribution, and changes in the quality or quantity of the lymphocyte population results in poor response to vaccination [28,29]. Based on our results, it is necessary to evaluate the immunogenicity for HBV at the diagnosis of IBD [30] and to minimize the time to vaccination.
Based on the immunologic principles of vaccinations, a positive relationship between a prior complete vaccination and vaccine response would be reasonable. However, our result showed a negative relationship. This negative relationship should be further analyzed based on the time of the primary vaccination and the serum titers of antibodies after primary vaccination, because there could be patients who failed to respond to the primary vaccination. Nearly 5% of individuals have been reported to show blunted immune response to vaccination [31]. However, nearly none of the patients in our study could recall that information.
Another explanation for this negative relationship could arise from the study design. We considered patients who were uncertain of a previous vaccination as a vaccine naïve group. Thus, some of these patients could have had a previous complete vaccination series. However, if we compare HBV vaccination rates from another Korean study, which reported a 52.6% rate, this kind of recall bias would be negligible. Fortunately, 77.2% of patients with a suboptimal response to the booster vaccine responded to an additional full series vaccine. Thus, a full series vaccination should be administered if the booster vaccination fails. Also, physicians should pay strict attention to this population of patients since a complete vaccination history is associated with poor compliance.
Immunosuppressive therapy, including conventional immunosuppressants such as corticosteroids or azathioprine, and biologics are closely related to suboptimal vaccine response [32,33]. The most recent American study also reported that exposure to anti-TNF, especially infliximab was associated with decreased antibody response [34] in IBD patients. However, in our study, there was no statistically significant association between the type of treatment and vaccine response although 70% of patients were under immunosuppressive therapy. These inconsistent results could be explained by different study design between 2 studies. Although the American study included relatively large number of patients, this study included patients with and without documented vaccination history which has significant influence on vaccine response as well as medication. In addition, the lack of an association between the treatment modality and response could be due to the mild disease activity of a majority of patients in our study. Therefore, we still have to monitor the negative influence of immunosuppressive agents carefully, especially the effects of anti-TNF agents on vaccine response, and consider appropriate vaccinations for non-immune patients before starting anti-TNF therapies [7,35,36].
This study has several limitations. First, as a single-center study, our subjects did not necessarily represent the characteristics of the general Korean IBD patient population. Second, we considered patients “not knowing about the vaccination” as non-vaccinated individuals. Some patients who had completed the vaccination, but could not recall having been vaccinated might be assigned to the full vaccination group. However, since the Korean National Immunization Program had started in 1995 and due to the relatively young age of enrolled patients, recall bias might not have significantly influenced the results. Despite these limitations, this study is valuable in terms of evaluating the status of immunogenicity against HBV in IBD patients within an intermediate endemic area and the vaccine response in subgroups classified as booster and vaccine naïve groups.
In conclusion, this study confirmed that IBD patients who are more susceptible to HBV infection have suboptimal response to an HBV vaccination. Thus, it is necessary to evaluate the immunogenicity to HBV at the diagnosis of IBD, and a vaccine schedule should be created as soon as possible. Considering that a previous complete HBV vaccination series is associated with suboptimal response and poor compliance, serum-HBV titers should be thoroughly evaluated in these patients, and, if negative, an additional 3-dose vaccination should be administered.
NOTES
Fig. 3.
Evaluation of vaccination response based on serum anti-HBs titer. AIR, adequate immune response defined as anti-HBs titer ≥10 IU/L; EIR, effective immune response defined as anti-HBs titer ≥100 IU/L.
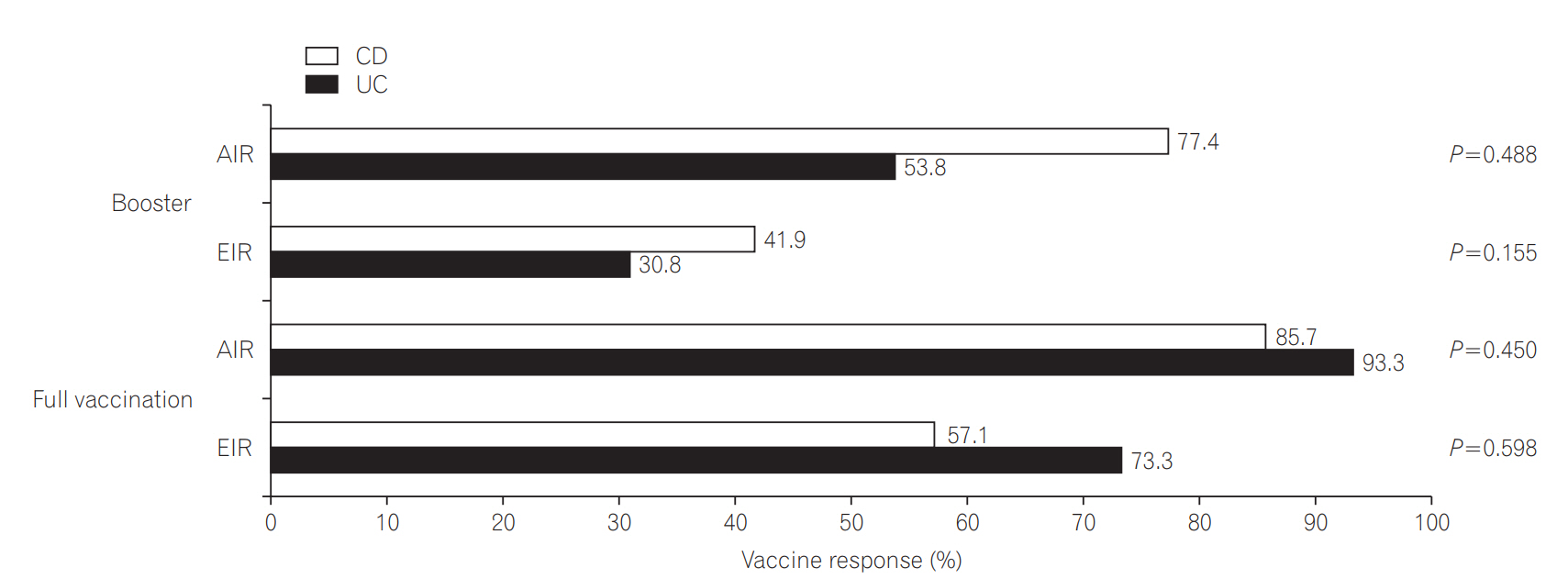
Fig. 4.
Vaccine response of complete 3-dose vaccination in the patients with inadequate response after booster vaccination.
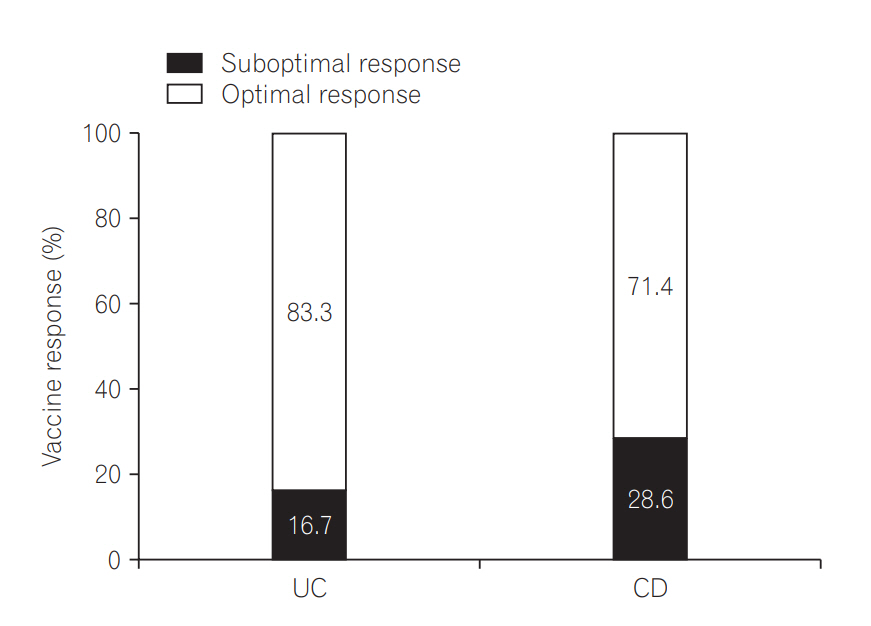
Table 1.
Baseline Characteristics of the Vaccinated Patients (n=73)
| Characteristics | Value |
|---|---|
| Age at the time of HBV vaccination (yr) | 29.9±12.3 |
| Disease duration (mo) | 56.8±61.6 |
| Male sex | 49 (67.1) |
| IBD subtype | |
| UC | 28 (38.4) |
| CD | 45 (61.6) |
| Disease severity | |
| Remission to mild | 60 (82.2) |
| Moderate to severe | 13 (17.8) |
| Type of treatment | |
| Biologics + immunomodulator | 10 (13.7) |
| Biologicsa | 13 (17.8) |
| Immunomodulatorb | 28 (38.4) |
| Non-immunomodatorc | 22 (30.1) |
| Comorbidity | |
| None | 67 (91.8) |
| Diabetes mellitus | 2 (2.7) |
| Hypertension | 3 (4.1) |
| Pancreatitis | 1 (1.4) |
| Indication for HBV vaccination | |
| Booster after complete HBV vaccination | 44 (60.3) |
| Incomplete vaccination or unknown history | 29 (39.7) |
Table 2.
Clinical Factors Predictive of Optimal Vaccination Response
| Variable | Total |
Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|---|
| Non-response | Optimala | P-value | OR | 95% CI | P-value | ||
| Age at HBV vaccination (yr)b | 0.798 | ||||||
| <26 | 34 (46.6) | 7 (20.6) | 27 (79.4) | 6.01 | 1.15-31.32 | 0.033 | |
| ≥26 | 39 (53.4) | 9 (23.1) | 30 (76.9) | (reference) | |||
| Sex | 0.656 | ||||||
| Male | 49 (67.1) | 10 (20.4) | 39 (79.6) | ||||
| Female | 24 (32.9) | 6 (25.0) | 18 (75.0) | ||||
| IBD type | 0.616 | ||||||
| UC | 28 (38.4) | 7 (25.0) | 21 (75.0) | ||||
| CD | 45 (61.6) | 9 (20.0) | 36 (80.0) | ||||
| Disease activity | 0.720 | ||||||
| Moderate to severe | 13 (17.8) | 2 (15.4) | 11 (84.6) | 2.09 | 0.32-13.53 | 0.441 | |
| Remission to mild | 60 (82.2) | 14 (23.3) | 46 (76.7) | (reference) | |||
| Previous HBV vaccination | 0.052 | ||||||
| Complete | 44 (60.3) | 13 (29.5) | 31 (70.5) | 0.15 | 0.03-0.80 | 0.026 | |
| Incomplete or unknown | 29 (39.7) | 3 (10.3) | 26 (89.7) | (reference) | |||
| Disease duration (mo) | 0.133 | ||||||
| <24 | 25 (34.2) | 8 (32.0) | 17 (68.0) | 0.28 | 0.06-1.19 | 0.084 | |
| ≥24 | 48 (65.8) | 8 (16.7) | 40 (83.3) | (reference) | |||
| Type of treatment | 0.765 | ||||||
| Biologics + immunomodulator | 10 (13.7) | 2 (20.0) | 8 (80.0) | 0.20 | 0.02-2.39 | 0.203 | |
| Biologicsc | 13 (17.8) | 2 (15.4) | 11 (84.6) | 0.66 | 0.07-5.81 | 0.706 | |
| Immunomodulatord | 28 (38.4) | 8 (28.6) | 20 (71.4) | 0.24 | 0.04-1.47 | 0.124 | |
| Non-immunomodatore | 22 (30.1) | 4 (18.2) | 18 (81.8) | (reference) | |||
Table 3.
Clinical Factors Associated with Poor Compliance of HBV Vaccination Recommendation
| Variable | Total |
Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|---|
| Good compliancea | Poor compliance | P-value | OR | 95% CI | P-value | ||
| Age at HBV vaccination (yr)b | 0.454 | ||||||
| <26 | 39 (44.8) | 34 (87.2) | 5 (12.8) | 2.74 | 0.41-18.28 | 0.299 | |
| ≥26 | 48 (55.2) | 39 (81.3) | 9 (18.8) | (reference) | |||
| Sex | >0.999 | ||||||
| Male | 59 (67.8) | 49 (83.1) | 10 (16.9) | ||||
| Female | 28 (32.2) | 24 (85.7) | 4 (14.3) | ||||
| IBD type | 0.752 | ||||||
| UC | 34 (39.1) | 28 (82.4) | 6 (17.6) | ||||
| CD | 53 (60.9) | 45 (84.9) | 8 (15.1) | ||||
| Disease activity | >0.999 | ||||||
| Moderate to severe | 15 (17.2) | 13 (86.7) | 2 (13.3) | 0.91 | 0.15-5.47 | 0.918 | |
| Remission to mild | 72 (82.8) | 60 (83.3) | 12 (16.7) | (reference) | |||
| Previous HBV vaccination | 0.029 | ||||||
| Complete | 48 (55.2) | 44 (91.7) | 4 (8.3) | 0.11 | 0.02-0.73 | 0.022 | |
| Incomplete or unknown | 39 (44.8) | 29 (74.4) | 10 (25.6) | (reference) | |||
| Disease duration (mo) | 0.607 | ||||||
| <24 | 32 (36.8) | 26 (81.3) | 6 (18.8) | 1.71 | 0.41-7.07 | 0.459 | |
| ≥24 | 55 (63.2) | 47 (85.5) | 8 (14.5) | (reference) | |||
| Type of treatment | 0.668 | ||||||
| Biologics + immunomodulator | 4x108 | 0 | 0.999 | ||||
| Biologicsc | 26 (29.9) | 23 (88.5) | 3 (11.5) | 0.79 | 0.13-4.68 | 0.797 | |
| Immunomodulatord | 33 (37.9) | 28 (84.8) | 5 (15.2) | 1.11 | 0.24-5.18 | 0.894 | |
| Non-immunomodulatore | 28 (32.2) | 22 (78.6) | 6 (21.4) | (reference) | |||
a Good compliance for the vaccination was defined as administration of all vaccination as recommend schedule.
c Biologics included infliximab, adalimumab, golimumab or any combination of these drugs with aminosalicylates.
REFERENCES
1. Altunöz ME, Senateş E, Yeşil A, Calhan T, Ovünç AO. Patients with inflammatory bowel disease have a lower response rate to HBV vaccination compared to controls. Dig Dis Sci 2012;57:1039-1044.


2. Huang ML, Xu XT, Shen J, Qiao YQ, Dai ZH, Ran ZH. Prevalence and factors related to hepatitis B and C infection in inflammatory bowel disease patients in China: a retrospective study. J Crohns Colitis 2014;8:282-287.



3. Gisbert JP, Chaparro M, Esteve M. Review article: prevention and management of hepatitis B and C infection in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2011;33:619-633.


4. Bernal I, Domènech E, García-Planella E, Cabré E, Gassull MA. Opportunistic infections in patients with inflammatory bowel disease undergoing immunosuppressive therapy. Gastroenterol Hepatol 2003;26:19-22.


5. Michel M, Duvoux C, Hezode C, Cherqui D. Fulminant hepatitis after infliximab in a patient with hepatitis B virus treated for an adult onset still’s disease. J Rheumatol 2003;30:1624-1625.

6. Wei SC, Chang TA, Chao TH, et al. Management of ulcerative colitis in Taiwan: consensus guideline of the Taiwan Society of Inflammatory Bowel Disease. Intest Res 2017;15:266-284.



7. Farraye FA, Melmed GY, Lichtenstein GR, Kane SV. ACG clinical guideline: preventive care in inflammatory bowel disease. Am J Gastroenterol 2017;112:241-258.



8. Moses J, Alkhouri N, Shannon A, et al. Hepatitis B immunity and response to booster vaccination in children with inflammatory bowel disease treated with infliximab. Am J Gastroenterol 2012;107:133-138.



9. Kim H, Shin AR, Chung HH, et al. Recent trends in hepatitis B virus infection in the general Korean population. Korean J Intern Med 2013;28:413-419.



10. Lee DH, Kim JH, Nam JJ, Kim HR, Shin HR. Epidemiological findings of hepatitis B infection based on 1998 National Health and Nutrition Survey in Korea. J Korean Med Sci 2002;17:457-462.



11. Lee H, Lee H, Cho Y, Oh K, Ki M. Changes in seroprevalence of hepatitis B surface antigen and epidemiologic characteristics in the Republic of Korea, 1998-2013. Epidemiol Health 2015;37:e2015055. doi: 10.4178/epih/e2015055.



13. López-Serrano P, Pérez-Calle JL, Sánchez-Tembleque MD. Hepatitis B and inflammatory bowel disease: role of antiviral prophylaxis. World J Gastroenterol 2013;19:1342-1348.



14. Ben Musa R, Gampa A, Basu S, et al. Hepatitis B vaccination in patients with inflammatory bowel disease. World J Gastroenterol 2014;20:15358-15366.



15. Lee YJ, Cheon JH, Kim JH, et al. Clinical efficacy of beclomethasone dipropionate in Korean patients with ulcerative colitis. Yonsei Med J 2017;58:144-149.


16. Sandborn WJ, Feagan BG, Hanauer SB, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology 2002;122:512-530.


17. Park NH, Chung YH, Lee HS. Impacts of vaccination on hepatitis B viral infections in Korea over a 25-year period. Intervirology 2010;53:20-28.


18. Kim ES, Cho KB, Park KS, et al. Prevalence of hepatitis-B viral markers in patients with inflammatory bowel disease in a hepatitis-B-endemic area: inadequate protective antibody levels in young patients. J Clin Gastroenterol 2014;48:553-558.


19. Yun HS, Min YW, Chang DK, et al. Factors associated with vaccination among inflammatory bowel disease patients in Korea. Korean J Gastroenterol 2013;61:203-208.


20. Coates T, Wilson R, Patrick G, André F, Watson V. Hepatitis B vaccines: assessment of the seroprotective efficacy of two recombinant DNA vaccines. Clin Ther 2001;23:392-403.


21. Poorolajal J, Hooshmand E. Booster dose vaccination for preventing hepatitis B. Cochrane Database Syst Rev 2016;(6): CD008256doi: 10.1002/14651858.CD008256.pub3.



22. Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis 2011;53:68-75.



23. Gisbert JP, Chaparro M. Vaccination strategies in patients with IBD. Nat Rev Gastroenterol Hepatol 2013;10:277-285.



24. Bruce MG, Bruden D, Hurlburt D, et al. Antibody levels and protection after hepatitis B vaccine: results of a 30-year follow-up study and response to a booster dose. J Infect Dis 2016;214:16-22.



25. Cossio-Gil Y, Martínez-Gómez X, Campins-Martí M, et al. Immunogenicity of hepatitis B vaccine in patients with inflammatory bowel disease and the benefits of revaccination. J Gastroenterol Hepatol 2015;30:92-98.


26. Fisman DN, Agrawal D, Leder K. The effect of age on immunologic response to recombinant hepatitis B vaccine: a metaanalysis. Clin Infect Dis 2002;35:1368-1375.



27. Vida Pérez L, Gómez Camacho F, García Sánchez V, et al. Adequate rate of response to hepatitis B virus vaccination in patients with inflammatory bowel disease. Med Clin (Barc) 2009;132:331-335.


29. Kumar R, Burns EA. Age-related decline in immunity: implications for vaccine responsiveness. Expert Rev Vaccines 2008;7:467-479.


30. Kim ES. Inflammatory bowel disease is no longer a risk factor of viral hepatitis infection in Asia. Intest Res 2017;15:5-6.



31. Walayat S, Ahmed Z, Martin D, Puli S, Cashman M, Dhillon S. Recent advances in vaccination of non-responders to standard dose hepatitis B virus vaccine. World J Hepatol 2015;7:2503-2509.



32. Agarwal N, Ollington K, Kaneshiro M, Frenck R, Melmed GY. Are immunosuppressive medications associated with decreased responses to routine immunizations? A systematic review. Vaccine 2012;30:1413-1424.


33. Gisbert JP, Villagrasa JR, Rodríguez-Nogueiras A, Chaparro M. Efficacy of hepatitis B vaccination and revaccination and factors impacting on response in patients with inflammatory bowel disease. Am J Gastroenterol 2012;107:1460-1466.



34. Pratt PK Jr, David N, Weber HC, et al. Antibody response to hepatitis B virus vaccine is impaired in patients with inflammatory bowel disease on infliximab therapy. Inflamm Bowel Dis 2018;24:380-386.



- TOOLS