 |
 |
- Search
| Intest Res > Volume 18(3); 2020 > Article |
|
See Editorial "Education levels and survival in colorectal cancer: is there really an obvious association?" in Volume 18 on page 247.
Abstract
Background/Aims
Colorectal cancer (CRC) is a public health problem. In Mexico, there have been no recent studies conducted on survival in terms of this pathology or on the influence of prognostic factors. The study aims to determine the probability of survival in patients with CRC presence of low levels of schooling and a rural population, adjusted for clinical stage and type of treatment.
Methods
A retrospective study was conducted in a cohort of 305 patients with CRC treated at State Cancer Center, located in Veracruz-Mexico; the follow-up period of 60 months (2012-2016). The survival probability was calculated using the Kaplan-Meier estimator and the log-rank test with 95% confidence intervals (CIs). Prognostic factors were determined using hazard ratio (HR) multivariate Cox regression analysis.
Results
Overall survival was 40% at 60 months. Subjects in the age group ≥ 65 years had a low survival rate of 28% (P=0.026) and an advanced clinical stage of 22% (P<0.001). Of the patients with bone metastasis, none survived longer than 5 years (P=0.008). With respect to the unfavorable prognostic factors identified in the multivariate analysis, a decreased level of schooling was associated with an HR of 7.6 (95% CI, 1.1-54.7), advanced clinical stage was associated with an HR of 2.1 (95% CI, 1.2-4.0), and the presence of metastasis had an HR of 1.8 (95% CI, 1.1-2.9).
Colorectal cancer (CRC) is a health problem worldwide. CRC is expected to increase due to the growth and aging of the population, as well as the adoption of behaviors and lifestyles that increase the risk of dying [1,2]. In Mexico, where CRC ranks fourth among the most common cancers, most cases are detected after 50 years of age, and the scenario is similar for both sexes. Therefore, the number of new cases and the mortality rates have increased consistently with the growing life expectancy, which currently presents a global public health problem [3].
Some sociodemographic characteristics have been studied and have been correlated with survival in several types of cancer, including CRC. Some studies have pointed out that survival is influenced by social inequalities [4-6]. Likewise, the socioeconomic stratum, which affects access to early detection programs and determines the type of health service available [7]. Similarly, lifestyle behaviors in rural area are considered risk factors for this type of cancer [8]. Some countries, such as Brazil, have reported an increase in the incidence of CRC in areas of unequal social stratum and low levels of schooling [9].
The important advances made in diagnostic and therapeutic techniques, together with prevention programs for early detection of malignancies in the preclinical phase, have contributed to improving the survival ratio [10,11]. Recent studies have shown that tumors located in the right colon are associated with lower survival compared to those restricted to the left colon, as shown by Loupakis et al. [12].
Long-term disease-free survival following the excision of polyps with metastasis observed due to the development in recent years of advanced surgical techniques. Differences in disease-free survival also depend on the chemotherapy regimen and planned cycles are completed [13]. Polyp resection is indicated when base is between 2 to 2.5 cm [13]. By colonoscopy it is possible to remove up to 70% of the polyps located in the colon and rectum [14].
Several randomized controlled trials conducted to compare the effectiveness of a series of treatment protocols [14,15]. Age and advanced stage colon cancer are considered predictive of early mortality, as well as disease characteristics such as having more than one positive lymph node, a poorly differentiated tumor and concomitant diseases [16]. Chemoradiation is the treatment of choice in tumors located in the rectum. The survival rate improved in patients with stage III colon cancer [17].
This study aimed to determine the probability of survival in patients with CRC and to identify the association between survival and low levels of schooling, low socioeconomic status, and location in a rural population, adjusting for the clinical and histologic stages of disease and the type of treatment.
A retrospective open cohort study was conducted in which all patients diagnosed in the 2010 to 2016 period were included in the State Cancer Center, a tertiary care center, located in Xalapa, Veracruz-Mexico. The maximum and the minimum follow-up period was 60 and 24 months, respectively and the patients were classified as exposed or not exposed according to the level of schooling. The selection criteria were patients with a confirmed diagnosis of CRC who were 18 years or older, of either both sex and who resided in the state of Veracruz. Participants were selected based on information obtained by secondary sources, such as clinical records and information provided by the Department of Social Work. The monitoring included the time interval between the date of diagnosis and the date of the last consultation or the date of death from any cause. For the rest of the cohort, the follow-up time ended on December 31, 2016. This study was approved by the Research Ethics Committee of State Cancer Center, Secretary of Health of the State of Veracruz (approval No. SEIC-006-16). This study is a retrospective study using medical record review and so informed consent was waived.
The main response variable was the survival of patients with CRC defined as the time between the confirmation of the diagnosis of CRC and the death of the patient. The death was verified through the death certificate provided by the social work coordination and by the records of the Epidemiological System and Death Statistics.
The predictor variables were a low level of schooling, defined as “less or equal” level of secondary schooling; socioeconomic status, defined as the number that indicates the social and economic position in which the patient’s family is located; and the rural population, defined as the population with less than 2,500 inhabitants.
Other covariables recorded were tumor size, histological type and clinical stage of disease using the criteria of the American Joint Commission on Cancer and the World Health Organization (WHO). Sociodemographic variables that were also included were the type of diagnosis and treatment, the date of the last contact and the date of death, as well as the presence of associated pathologies. The criteria for socioeconomic classification were the following variables with their respective weight: family income (55%), job (10%), family expenses (10%), housing (20%), and family health (5%).
Experts from the study’s headquarters hospital and the Instituto Nacional de Oncología, located in Havana, Cuba, validated the collection card. The following information collected through the clinical file: diagnosis of CRC (histological tissue and colonoscopy). The date and cause of death were obtained from the death certificate.
The size of the sample was calculated from the results of a cohort study by Lejeune et al. [18], where it was found that the survival of the subjects with a low level of education was 57.1% (HR, 0.73; 95 CI, 0.57-0.94). With the information mentioned above and considering a confidence level of 95% and a minimum power of 80%, the sample size calculated was 104 patients.
Descriptive statistics were used for sociodemographic, clinical, and histological characteristics and those related to the treatment of the studied cohort. The survival analysis was calculated with the Kaplan-Meier method. Survival probabilities were compared for each possible prognostic factor using the log-rank test. Subsequently, the prognostic factors adjusted for possible confounders in a multivariate analysis using Cox regression, where the dependent variable was age, and the covariates were clinical stage, histological type, level of schooling, place of residence. The HRs and their respective 95% CIs were calculated. Losses during the monitoring were considered censored data and were contributed to the sum of the total person-time. The statistical package IBM SPSS statistics software (IBM Corp., Armonk, NY, USA), for Windows version 23.0, was used for analysis.
Of the 323 patients initially considered, the following were excluded: 5 resided in another geographical area, 8 had recurrent CRC, and 5 had another type of cancer added to the primary malignancy. In total, the study sample consisted of 305 patients, mostly men, with a minimum and maximum age of 18 and 96 years, respectively. The population from rural areas predominated, had an educational level equal to or lower than the basic level, had mostly paid employment, and almost the entire population was registered at a low socioeconomic status (Table 1).
The methods used to diagnose suspicion of CRC were, 66% colonoscopy, 20.5% imaging tests and 13.5% surgery. The frequency tumors stages were II 29%, III 30.6%, and IV 33.3%, principally located in the rectum and were greater than 5 centimeters. The liver was the organ most frequently affected by distant metastasis, and adenocarcinoma was the most common histological type (Table 2).
The most frequent symptoms were colic (49.2%), combined with weight loss and rectal bleeding (43.6%), among other symptoms. The most frequent types of comorbidities were arterial hypertension (34.8%) and diabetes mellitus (28.3%). The most frequent treatment undergone was a combination of surgery with chemotherapy, as well as chemotherapy with oxaliplatin (Table 2).
The overall survival ratio for the cohort at 60 months of monitoring was 40%. A greater statistically significant survival was observed in patients >65 years of age (P=0.03) who had a high level of education (P=0.02) (Table 3). Those patients with an early clinical stage (P<0.001) and without metastasis (P<0.001) had a high probability of survival (Table 4). When evaluating the types of treatment, when a combination included surgical treatment, survival was 41% (P=0.012) (Fig. 1).
After adjusting for age (<65 years and ≥65 years), the following variables showed a significant association with survival: low level of schooling, tumors in an advanced clinical stage and the presence of metastasis, all of which increased the risk of dying due to poor prognostic factors (Table 5).
There is a wide variation in survival between different geographical areas; due to differences in exposure to risk factors for CRC, access to cancer diagnosis and type of treatment. As shown in our results, the 5-year global survival was very low, similar to that reported in other Latin American countries, such as Chile and Colombia [19,20].
Similarly, these inconsistencies in survival attributed to the inequalities in socioeconomic status and the geographic area of origin [9]. Even the low level of schooling related to an increased risk of CRC [21]. A low 37% survival in the population was associated with a low level of education, which, in the multivariate analysis, was a poor prognostic factor (HR, 7.46; 95% CI, 1.02-54.7) in our cohort study. Similar results were reported by Cavalli-Björkman et al. [22]. In contrast, a recent study suggested that a high level of schooling was a protective factor against CRC (HR, 0.61; 95% CI, 0.39-0.92), compared to illiterate patients [23]. Additionally, the education level was associated with the histological type of CRC and with the age of the subject in that, the young people developed aggressive tumors according to the histological differentiation [24,25].
The clinical stage is still considered an element that can guide therapeutic treatment and constitutes a prognostic factor in patients [26]. In the study in general, more than 60% of tumors diagnosed in advanced stages, implied a lower survival and a higher risk of dying from CRC. In recent reports, the International Agency for Research on Cancer showed a high incidence of advanced stages of CRC not only in developing countries but also in developed countries [1]. This is the case in Mexico and specifically for the cohort under study with a high degree of marginalization. In agreement, other studies conducted in populations of low socioeconomic status or located in remote areas showed a high incidence of advanced stage disease, revealing the lack of early detection programs and, in some contexts, poorer standards of care and difficulty in accessing screening tests [1,9,23,26].
In addition, the same effect observed in our results of advanced stage disease: a very low survival of 22%, unlike the rate of survival in the early stages (68%), similarity with what has been reported for this type of cancer [27,28]. These data indicate CRC diagnosis at late stage, reducing the probability of survival, which suggests the need to increase efforts for detection in early stages.
In the multivariate analysis (Table 5), stages III and IV emphasized as poor prognostic factors with an HR of 2.24 (1.15-4.36), which coincides with the findings by Sharkas et al. [27] who reported, when subdividing the tumor into regional and distant metastasis (HR, 1.8; 95% CI, 1.6-3.7; HR, 4.5; 95% CI, 3.7-5.1, respectively).
Our results, consistent with the current literature, have shown that the presence of metastasis shown to be an important predictor of recurrence and poor survival and was the main cause of mortality in patients with CRC [29]. We observed that a significant number of these patients experienced early metastasis in the liver, followed by metastasis to the bone and the lung. Due to the location, size, and extent of metastasis, no patient survived after 5 years. The prognosis and clinical characteristics of bone metastasis in CRC poorly studied due to their low incidence and insufficient research [30,31].
The main factor linked to improved survival in CRC, according to the literature, is the progress that made with adjuvant treatments after surgery [32,33]. While undergoing some surgical procedures they showed a better effect on survival (47%) than not undergoing surgery (27%) (P=0.002) (Fig. 1E). However, 5-fluorouracil monotherapy was the treatment with the highest survival rates. Surgery in this type of neoplasm is very invasive, and the older the patient, the greater the risk of developing complications, which is also associated with the patient’s living conditions.
In contrast, we identified that the combination of surgical treatment and chemotherapy showed the best survival at 38%. However, another study showed a survival of only 58.3% with surgery, and survival in patients who received adjuvant chemotherapy increased to 83.4% [33]. Analogous results reported for localized tumors that were operated on and treated with 5FU postoperatively, and they showed a great benefit in stage III [32]. The studies described previously conducted in developed countries, such as Japan and the United States, where prevention programs implemented and living conditions differ from those of our study cohort [33].
CRC represents a large global burden that predicted to increase due to the growth and aging of the population because of the adoption of behaviors and lifestyles that increase the risk of developing CRC. By recognizing this growing problem in Mexico and implementing consistent monitoring (pre- and postsurgical), the poor survival associated with CRC and the rate of recurrence could help spur efforts to optimize management by increasing the availability of effective access to timely detection. Intensifying educational protocols in the management and control of CRC risk factors and disseminating information that will trigger a social response in accordance with the available conditions and resources.
Finally, the predictor with the highest association to low survival was “less or equal” to the level of secondary schooling (HR, 7.6; 95% CI, 1.1-54.7), which was independent of other covariates, such as occupation or profession and economic income; the latter was not analyzed since all patients reported the lowest economic level. However, other indicators were included that gave a good approximation of the socioeconomic status.
Implementing a screening program and developing additional strategies based on the population at risk of CRC would reduce the gap between the survival of patients with high and low schooling, as well as decrease the incidence of CRC in the coming years. In addition, these strategies could reduce the degree of disease progression in preclinical and clinical patients, thus accelerating treatment and reflecting favorable changes in survival.
The strengths of the present study were the inclusion of the sample size, the collection of standardized data and a limited loss during follow-up. Information bias was reduced by corroborating all clinical records. An approved criterion when making multidisciplinary decisions. The main limitations of this study include the retrospective nature of this study and the restrictions inherent to the quality of the information in the clinical records that belong to the heterogeneous population and the use of different diagnostic methods. Our study had another limitation: it included a percentage of unclassified patients from a public institution.
In conclusion, regarding the variables that influenced survival, the presence of metastasis, advanced disease and low education were determined to be predictive in more than half of the cases studied that were diagnosed in advanced stages (III-IV) and had a lower overall survival. Possible explanations include late diagnosis, barriers to timely access to care and effective treatment and underlying comorbidities.
NOTES
FINANCIAL SUPPORT
The authors received no financial support for the research, authorship, and/or publication of this article.
AUTHOR CONTRIBUTION
Conceptualization: Alvarez-Bañuelos MT, Quezada-Gutiérrez C, Guzmán-García RE. Methodology: Alvarez-Bañuelos MT, Quezada-Gutiérrez C, Morales-Romero J. Validation: AlvarezBañuelos MT, Sampieri CL, Monte-Villaseñor E. Formal analysis: Morales-Romero J, Quezada-Gutiérrez C. Investigation: Quezada-Gutiérrez C, Alvarez-Bañuelos MT. Writing-original draft preparation: Quezada-Gutiérrez C, Alvarez-Bañuelos MT. Writing-review and editing: Sampieri CL, Morales-Romero J, Guzmán-García RE. Supervision: Alvarez-Bañuelos MT, Morales-Romero J, Monte-Villaseñor E. Approval of final manuscript: all authors.
ACKNOWLEDGEMENTS
This study did not receive any type of funding but received the support of the Public Health Institute of the University of Veracruz, which played a key role in the design and conduct of the study, collection, management, analysis and interpretation of the data, the preparation, the revision and the approval of the manuscript. The Research and Teaching Area and the archives of the Veracruz State Cancer Center also supported the present study.
Fig. 1.
Kaplan-Meier curves of overall survival colorectal cancer. (A) Age, (B) level of education, (C) clinical stage, (D) metastatic, (E) treatment with surgery, and (F) treatment with chemotherapy.
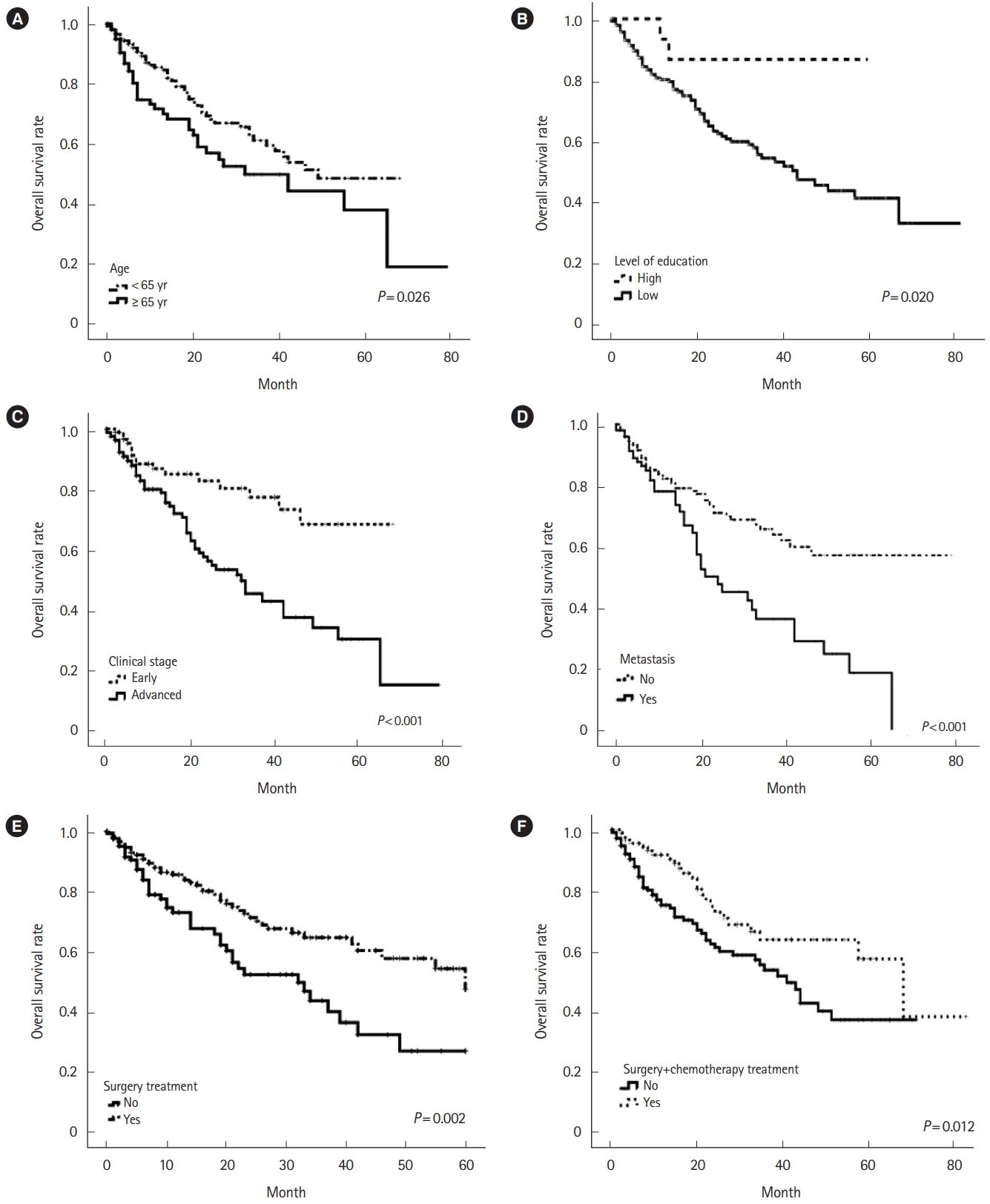
Table 1.
Sociodemographic and Clinical Characteristics of the Cohort Participants (n=305)
| Variable | No. of patients (%) |
|---|---|
| Age (yr) | |
| < 65 | 208 (68.2) |
| ≥ 65 | 97 (31.8) |
| Sex | |
| Male | 178 (58.4) |
| Female | 127 (41.6) |
| Place of origin | |
| Rural | 196 (64.3) |
| Urban | 109 (35.7) |
| Occupation | |
| Worker | 163 (53.4) |
| Housework | 116 (38.1) |
| Unemployed | 26 (8.5) |
| Clinical stagea | |
| Local | 132 (51.2) |
| Regional | 40 (15.5) |
| Distant | 86 (33.3) |
| Level of education | |
| Illiterate | 132 (43.3) |
| Primary/secondary education | 152 (49.8) |
| High school/university | 21 (6.9) |
| Location of the tumor | |
| Blind | 40 (13.1) |
| Ascending+descending colon | 81 (26.6) |
| Transverse colon | 22 (7.2) |
| Sigmoid | 52 (17.0) |
| Straight | 110 (36.1) |
| Tumor size (cm) | |
| ≤5 | 97 (34.8) |
| > 5 | 182 (65.2) |
| Metastatic lymph nodes | |
| No | 250 (82.0) |
| Yes | 55 (18.0) |
| Location of metastasis | |
| Liver | 41 (41.4) |
| Lung | 15 (15.2) |
| Bone | 5 (5.0) |
| Others | 38 (38.4) |
Table 2.
Sociodemographic and Clinical Characteristics of the Cohort Participants
| Variable |
Education |
P-valuea | |
|---|---|---|---|
| Low (n=284) | High (n=21) | ||
| Age (yr) | 0.070 | ||
| < 65 | 190 (66.9) | 18 (87.5) | |
| ≥ 65 | 94 (33.1) | 3 (14.3) | |
| Sex | 0.170 | ||
| Male | 167 (58.8) | 11 (52.4) | |
| Female | 117 (41.2) | 10 (47.6) | |
| Place of origin | 0.002 | ||
| Rural | 189 (66.5) | 7 (33.3) | |
| Urban | 95 (33.5) | 14 (66.7) | |
| Occupation | 0.730 | ||
| Worker | 151 (49.5) | 12 (57.1) | |
| Unemployed | 55 (50.5) | 9 (42.9) | |
| Clinical stage | 0.960 | ||
| Early | 87 (30.6) | 6 (28.6) | |
| Advanced | 153 (53.9) | 12 (57.5) | |
| Unclassified | 44 (15.5) | 3 (14.3) | |
| Location of the tumor | 0.230 | ||
| Blind | 38 (13.5) | 2 (9.5) | |
| Ascending+descending colon | 77 (27.3) | 4 (19.0) | |
| Transverse colon | 17 (7.4) | 4 (19.0) | |
| Sigmoid | 47 (16.7) | 4 (19.0) | |
| Straight | 103 (36.5) | 7 (33.3) | |
| Tumor size (cm) | 0.190 | ||
| 2-5 | 93 (35.8) | 4 (21.1) | |
| > 5 | 167 (64.2) | 15 (78.9) | |
| Metastatic lymph nodes | < 0.001 | ||
| No | 239 (84.2) | 11 (52.4) | |
| Yes | 45 (15.8) | 10 (47.6) | |
| Location of metastasis | 0.030 | ||
| Liver | 40 (41.7) | 1 (33.3) | |
| Lung | 13 (13.5) | 2 (66.7) | |
| Others | 43 (44.8) | 0 | |
| Type of treatment (n = 261) | 0.170 | ||
| Surgery | 18 (18.2) | 1 (5.0) | |
| Chemotherapy | 23 (23.2) | 5 (25.0) | |
| Radiotherapy | 1 (1.0) | 1 (5.0) | |
| Surgery and chemotherapy | 37 (37.4) | 10 (50.0) | |
| Surgery and radiotherapy | 3 (3.0) | 1 (5.0) | |
| Chemotherapy and radiotherapy | 7 (7.1) | 1 (5.0) | |
| Surgery+chemotherapy+radiotherapy | 10 (10.1) | 1 (5.0) | |
| Type of chemotherapy (n = 182) | 0.590 | ||
| Neoadjuvant | 61 (33.5) | 5 (27.8) | |
| Adjuvant | 121 (66.5) | 13 (72.2) | |
| Scheme of chemotherapy (n = 163) | 0.960 | ||
| Oxaliplatin | 59 (39.6) | 5 (35.7) | |
| XELOX | 37 (24.8) | 4 (28.6) | |
| FOLFOX | 15 (10.1) | 2 (14.3) | |
| 5 FU | 20 (13.4) | 16 (14.3) | |
| Others | 18 (12.1) | 1 (7.1) | |
Table 3.
Survival According to Sociodemographic Characteristics (n=305)
| Variable | No. of subjects | 2-Year survival rate (%)a | 5-Year survival rate (%)a | P-valueb |
|---|---|---|---|---|
| Age (yr) | 0.03 | |||
| < 65 | 208 | 68 | 48 | |
| ≥ 65 | 97 | 56 | 28 | |
| Sex | 0.62 | |||
| Male | 178 | 67 | 44 | |
| Female | 127 | 61 | 38 | |
| With partner or spouse | 0.89 | |||
| Yes | 111 | 65 | 45 | |
| Do not | 194 | 63 | 33 | |
| Provenance zone | 0.85 | |||
| Urban | 109 | 65 | 43 | |
| Rural | 196 | 63 | 39 | |
| Level of education | 0.02 | |||
| Low | 284 | 62 | 37 | |
| High | 21 | 86 | 86c | |
| Employment situation | 0.34 | |||
| Worker | 163 | 70 | 42 | |
| Unemployed | 142 | 58 | 36 | |
| Socioeconomic level | NAd | |||
| Low | 295 | 63 | 38 | |
| High | 10 | 99.9 | 99.9 |
Table 4.
Survival According to Tumor Characteristics and Treatment
| Variable | No. | 2-Year survival rate (%)a | 5-Year survival rate (%)a | P-valueb |
|---|---|---|---|---|
| Clinical stage | < 0.001 | |||
| Early | 93 | 82 | 68 | |
| Advanced | 165 | 56 | 22 | |
| Tumor location | 0.700 | |||
| Descendent colon | 21 | 75 | 75 | |
| Blind | 40 | 75 | 55c | |
| Transverse colon | 21 | 63 | 50d | |
| Rectum | 110 | 61 | 46 | |
| Ascendant colon | 60 | 60 | 33 | |
| Sigmoid colon | 51 | 84 | 19 | |
| Tumor size (cm) | 0.700 | |||
| < 2 | 11 | 60 | 45e | |
| 2-5 | 86 | 66 | 49 | |
| > 5 | 182 | 65 | 39 | |
| Metastatic lymph node | 0.110 | |||
| 0 | 249 | 60 | 42 | |
| 1-3 | 29 | 68 | 34f | |
| ≥ 4 | 26 | 88 | 0 | |
| Presence metastasis | < 0.001 | |||
| Negative | 204 | 71 | 57 | |
| Positive | 101 | 47 | 0 | |
| Distant metastases | 0.008 | |||
| Lung | 15 | 74 | 48g | |
| Liver | 41 | 24 | 0h | |
| Lung and liver | 9 | 0 | 0 | |
| Bone | 5 | 53 | 0 | |
| Others | 29 | 57 | 0 | |
| Histologic grade | 0.170 | |||
| Grade 1 | 195 | 24 | 41 | |
| Grade 2 | 68 | 70 | 39 | |
| Grade 3 | 32 | 50 | 37i | |
| Type of chemotherapy | 0.150 | |||
| Adjuvant | 61 | 61 | 34 | |
| Neoadjuvant | 121 | 70 | 40 | |
| Scheme of chemotherapy | 0.320 | |||
| 5 FU | 22 | 73 | 63 | |
| Capecitabine | 16 | 57 | 57 | |
| Oxaliplatin | 64 | 70 | 43 | |
| FOLFOX | 17 | 62 | 31 | |
| XELOX | 41 | 68 | 0 | |
| Others | 3 | 50 | 0 |
Table 5.
Multivariate Analysis in Variables of Factors Associated with the Survival of Colorectal Cancer
REFERENCES
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424.


2. Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177-193.


3. Estadísticas a propósito del… día mundial contra el cancer: datos nacionales. National Institute of Statistics and Geography Web site. https://www.inegi.org.mx/contenidos/saladeprensa/aproposito/2018/cancer2018_nal.pdf. Updated February 2, 2018. Accessed March 5, 2020.
4. Le H, Ziogas A, Lipkin SM, Zell JA. Effects of socioeconomic status and treatment disparities in colorectal cancer survival. Cancer Epidemiol Biomarkers Prev 2008;17:1950-1962.


6. Fleming ST, Mackley HB, Camacho F, et al. Clinical, sociodemographic, and service provider determinants of guideline concordant colorectal cancer care for Appalachian residents. J Rural Health 2014;30:27-39.


7. Lai Y, Wang C, Civan JM, et al. Effects of cancer stage and treatment differences on racial disparities in survival from colon cancer: a United States population-based study. Gastroenterology 2016;150:1135-1146.



8. Beckmann KR, Bennett A, Young GP, et al. Sociodemographic disparities in survival from colorectal cancer in South Australia: a population-wide data linkage study. BMC Health Serv Res 2016;16:24.




9. Parreira VG, Meira KC, Guimarães RM. Socioeconomic differentials and mortality from colorectal cancer in large cities in Brazil. Ecancermedicalscience 2016;10:614.


10. Buie WD, Attard JA. Follow-up recommendations for colon cancer. Clin Colon Rectal Surg 2005;18:232-243.




11. Van Cutsem E, Borràs JM, Castells A, et al. Improving outcomes in colorectal cancer: where do we go from here? Eur J Cancer 2013;49:2476-2485.


12. Loupakis F, Yang D, Yau L, et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst 2015;107-dju427.


13. Pruitt SL, Harzke AJ, Davidson NO, Schootman M. Do diagnostic and treatment delays for colorectal cancer increase risk of death? Cancer Causes Control 2013;24:961-977.




14. Hurwitz HI, Tebbutt NC, Kabbinavar F, et al. Efficacy and safety of bevacizumab in metastatic colorectal cancer: pooled analysis from seven randomized controlled trials. Oncologist 2013;18:1004-1012.



15. Ciani O, Buyse M, Garside R, et al. Meta-analyses of randomized controlled trials show suboptimal validity of surrogate outcomes for overall survival in advanced colorectal cancer. J Clin Epidemiol 2015;68:833-842.


16. Neugut AI, Matasar M, Wang X, et al. Duration of adjuvant chemotherapy for colon cancer and survival among the elderly. J Clin Oncol 2006;24:2368-2375.


17. Ling CR, Wang R, Wang MJ, Ping J, Zhuang W. Prognosis and value of preoperative radiotherapy in locally advanced rectal signet-ring cell carcinoma. Sci Rep 2017;7:45334.




18. Lejeune C, Sassi F, Ellis L, et al. Socio-economic disparities in access to treatment and their impact on colorectal cancer survival. Int J Epidemiol 2010;39:710-717.



19. Mondaca S, Villalón C, Leal JL, et al. Benefit of adjuvant 5-fluorouracil based chemotherapy for colon cancer: a retrospective cohort study. Rev Med Chil 2016;144:145-151.


20. Armando C, Bravo LE, Clín P, García LS, Collazos P. Colorectal cancer incidence, mortality and survival in Cali, Colombia, 1962-2012. Salud Publica Mex 2014;56:457-464.


21. Leufkens AM, Van Duijnhoven FJ, Boshuizen HC, et al. Educational level and risk of colorectal cancer in EPIC with specific reference to tumor location. Int J Cancer 2012;130:622-630.


22. Cavalli-Björkman N, Lambe M, Eaker S, Sandin F, Glimelius B. Differences according to educational level in the management and survival of colorectal cancer in Sweden. Eur J Cancer 2011;47:1398-1406.


23. Rasouli MA, Moradi G, Roshani D, Nikkhoo B, Ghaderi E, Ghaytasi B. Prognostic factors and survival of colorectal cancer in Kurdistan province, Iran: a population-based study (2009-2014). Medicine (Baltimore) 2017;96:e5941.



24. Sonnenberg A, Turner KO, Genta RM. Ethnic variations in the occurrence of colonic neoplasms. United European Gastroenterol J 2017;5:424-431.


25. Kim TJ, Kim ER, Hong SN, Chang DK, Kim YH. Long-term outcome and prognostic factors of sporadic colorectal cancer in young patients: a large institutional-based retrospective study. Medicine (Baltimore) 2016;95:e3641.



26. Robbins AS, Siegel RL, Jemal A. Racial disparities in stage-specific colorectal cancer mortality rates from 1985 to 2008. J Clin Oncol 2012;30:401-405.


27. Sharkas GF, Arqoub KH, Khader YS, et al. Colorectal cancer in Jordan: survival rate and its related factors. J Oncol 2017;2017:3180762.




28. White A, Joseph D, Rim SH, Johnson CJ, Coleman MP, Allemani C. Colon cancer survival in the United States by race and stage (2001-2009): findings from the CONCORD-2 study. Cancer 2017;123 Suppl 24(Suppl 24): 5014-5036.


29. Ryuk JP, Choi GS, Park JS, et al. Predictive factors and the prognosis of recurrence of colorectal cancer within 2 years after curative resection. Ann Surg Treat Res 2014;86:143-151.



30. Neuman HB, O’Connor ES, Weiss J, et al. Surgical treatment of colon cancer in patients aged 80 years and older: analysis of 31,574 patients in the SEER-Medicare database. Cancer 2013;119:639-647.


31. Liu F, Zhao J, Xie J, et al. Prognostic risk factors in patients with bone metastasis from colorectal cancer. Tumour Biol 2016;37:16127-16134.


- TOOLS








