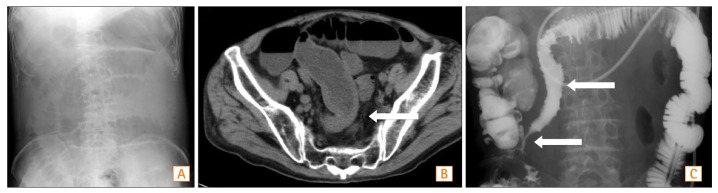
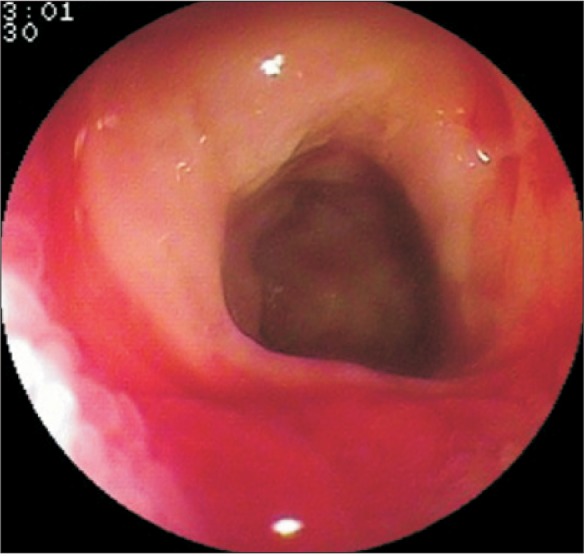
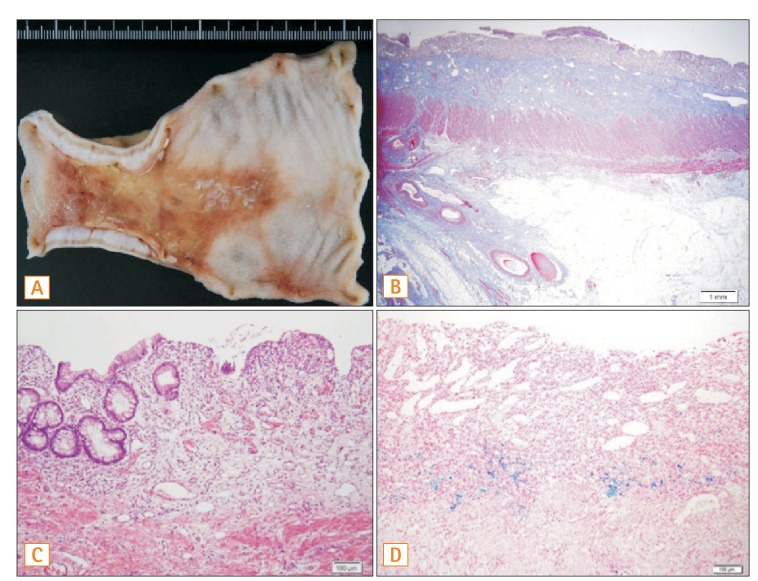
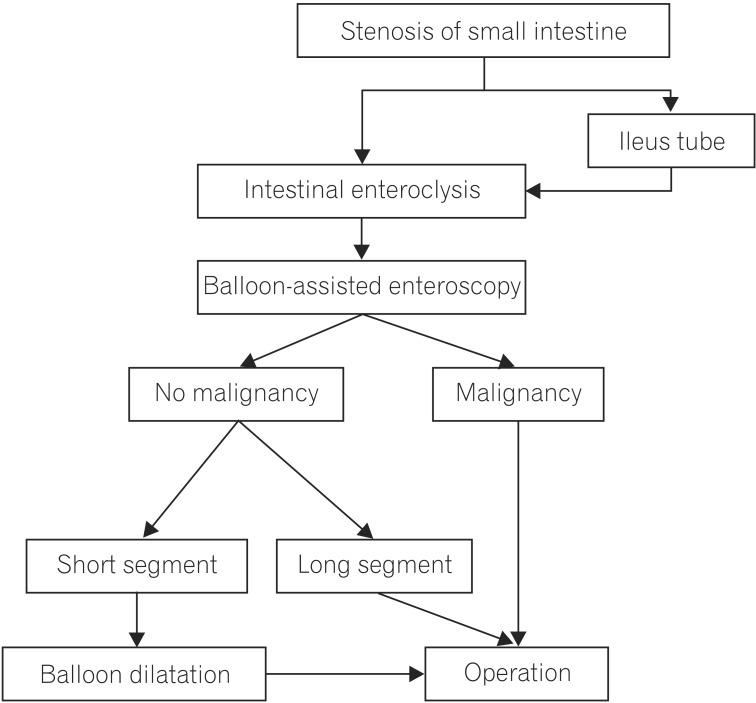
1. Medina C, Vilaseca J, Videla S, Fabra R, Armengol-Miro JR, Malagelada JR. Outcome of patients with ischemic colitis: review of fifty-three cases. Dis Colon Rectum 2004;47:180-184.PMID:
15043287.


2. Iwashita A, Yao T, Iida M, Yao T, Fuchigami T, Toda T. Clinicopathologic study on ischemic stricture of the small intestine. Stomach Intest 1990;25:557-569.
3. R├żf LE. Ischaemic stenosis of the small intestine. Acta Chir Scand 1969;135:253-259.PMID:
5803968.

4. Sada M, Kobayashi K, Takeuchi H, et al. Ischemic enteritis. Stomach Intest 2008;43:617-623.
5. Nakayama H, Kashiwagi Y, Nishizawa M, Ogawa K, Okui K. A case of ischemic stenosis of small intestine. J Jpn Soc Coloproctol 1997;50:249-253.

6. Saito T, Suzuki T, Watanabe K, et al. A case of ischemic stenosis of the small intestine revealed by double balloon endoscopy. Prog Dig Endosc 2010;77:104-105.

7. Gillet M, Philippe E, Stoebner P, Sava G, Viville C, Grenier JF. Cicatricial stenosis of the small intestine of ischemic origin: 7 cases. Ann Chir 1969;23:481-491.PMID:
5376386.

8. Takeuchi N, Naba K. Small intestinal obstruction resulting from ischemic enteritis: a case report. Clin J Gastroenterol 2013;6:281-286.PMID:
23990850.



9. Ohtaka H, Katsumata T, Takemiya M, et al. A case of ischemic stricture of the small intestine associated with Buerger's disease. Nihon Shokakibyo Gakkai Zasshi 1984;81:2588-2593.PMID:
6527425.

10. Yamanaka H, Matsuda S, Suzuki H. Two resected cases of stenotic type ischemic jejunoileitis. J Jpn Surg Assoc 1999;60:736-741.

11. Masuda K, Yamamoto T, Shida H, Ban K, Imanari T, Machida T. Ischmic enteritis causing a small bowel stenosis ŌĆöReport of a caseŌĆö. J Jpn Surg Assoc 1996;57:2227-2231.

12. Iwakiri Y, Nakamura K, Sasaki T, et al. Two cases of ischemic enteritis. Jpn J Clin Exp Med 1996;73:1365-1370.
13. Hidaka K, Kobayashi K, Kokubu S. AN operated case of ischemic stricture of the small intestine complicated with extrahepatic portal occlusion. Nihon Shokakibyo Gakkai Zasshi 1997;94:839-844.PMID:
9436392.

14. Hagimoto T, Ohdera K, Nakata K, et al. Serial radiographic change of ischemic enteritis ŌĆöReport of a caseŌĆö. J Jpn Soc Coloproctol 1999;52:505-511.

15. Nakamura F, Dohke M, Narita Y, Miyazaki K, Matsunami O, Katoh H. A case of ischemic stricture of the small intestine. J Jpn Surg Assoc 1999;60:129-133.

16. Watanabe T, Hirano M, Kitajima K, Okita K, Yarimizu T, Akizuki S. A case of ischemic enteritis showing a tubular stenosis. Nihon Shokakibyo Gakkai Zasshi 2000;97:191-194.PMID:
10707589.

17. Hada T, Kohno S, Oda Y, Kobayashi I, Ikegami M, Yamazaki Y. Resection and stricturoplasty for ischemic enteritis: a case report. Jpn J Gastroenterol Surg 2000;33:1831-1834.

18. Mukogawa T, Okumura T, Sugimori S, Misaki S, Nakano H. Ischemic stenosis of the small intestine ŌĆöReport of a caseŌĆö. J Jpn Surg Assoc 2000;61:675-679.

19. Higashi Y, Nakamura T, Hayashi T, Uno A, Konno H, Nakamura S. A case of ischemic stenosis of the small bowel treated with laparoscopy-assisted surgery. J Jpn Surg Assoc 2004;65:1277-1280.

20. Kanari M, Fujisawa J, Yukawa N, Nagano A, Matsukawa H, Kawano N. A case of ischemic stenosis of the small intestine. J Jpn Surg Assoc 2006;67:2396-2399.

21. Kashizuka H, Yamamoto M, Nishiwaki H, Hosoi T, Tsutsumi M, Imagawa A. A case of stenotic type ischemic enteritis with hepatic portal venous gas. Jpn J Gastroenterol Surg 2006;39:1850-1855.

22. Tokura M, Uesaka K, Seima Y, Fujiwara S, Shimada Y. A case of ischemic stricture of the small intestine which could be observed with colonoscopy. J Jpn Surg Assoc 2007;68:2778-2782.

23. Kan H, Furukawa K, Suzuki H, et al. A case of ischemic stenosis of the small intestine revealed by double balloon enteroscopy and resected by laparoscopy-assisted surgery. Jpn J Gastroenterol Surg 2007;40:1514-1519.

24. Unotoro J, Takita N, Suzuki Y, Shiozaki T, Tamazaki S, Tsurumaru M. A case of ischemic stricture of the jejunum due to ischemic enteritis. J Jpn Surg Assoc 2008;69:385-389.

25. Ohhara M, Miyake H, Kikuchi T, Hara J, Kimizuka K. A case of ischemic stenosis of the small intestine associated with ischemic colitis of the sigmoid colon. J Jpn Surg Assoc 2008;69:1964-1967.

26. Gohongi T, Ogata T, Nakano Y, Iida H, Gunji N, Orii K. A case of ileus caused by ischemic enteritis of the ileum. J Jpn Surg Assoc 2009;70:2013-2016.

27. Uematsu J, Kawai T, Yamamoto K, et al. A case of ischemic enteritis in young age. Prog Dig Endosc 2010;76:86-87.

28. Naito M, Haneda T, Ishiguro T. A case of ischemic enteritis diagnosed by exploratory surgery. Hokkaido J Surg 2010;55:143-146.
29. Iinuma N, Yamamoto K, Kubota K, Takagi S. A case of ischemic stenosis of the small intestine. Shinshu Med J 2012;60:365-370.
30. Takago S, Morishita M, Matsunoki A, Arano Y, Iida S, Minato H. A case of stricture type ischemic ileitis associated with ischemic colitis. J Jpn Surg Assoc 2013;74:1538-1542.