 |
 |
- Search
| Intest Res > Volume 15(2); 2017 > Article |
|
Abstract
Background/Aims
Infliximab has proven to be effective in the treatment of perianal fistulas in Crohn's disease (CD) but the efficacy of adalimumab is still unclear. The aim of this study is to assess the clinical efficacy of adalimumab and compare the results with those for infliximab.
Methods
Forty-seven CD patients treated for perianal fistulas with infliximab from September 2005 to December 2010 (n=31), or with adalimumab from November 2010 to May 2012 (n=16), were enrolled in this retrospective study. The following patient characteristics were analyzed; intestinal lesion site, fistula classification, seton placement, index of inflammatory bowel disease, C-reactive protein level, follow-up period, and the cumulative rate of nonrecurrence or aggravation of fistula.
Results
There were no significant differences in the intestinal lesion site, fistula classification, inflammatory bowel disease index, C-reactive protein level, and the frequency of injection between the infliximab group and the adalimumab group. The cumulative rate of nonrecurrence or aggravation of fistula was 62.5% in the adalimumab group and 83.9% in the infliximab group at 24 months after treatment (P=0.09). The risk factors for recurrence or aggravation may be related to seton placement (P=0.02), gender (P=0.06), and fistula classification (P=0.07).
Conclusions
There was no significant difference in the clinical efficacy of adalimumab and infliximab in the treatment of perianal fistulas in CD. However, fistula classification may be an important risk factor for recurrence or aggravation. The preliminary findings in this study show that further research is warranted.
The incidence of CD is higher in Western industrialized countries. The prevalence of CD in children younger than 20 years of age is 43 (95% CI, 40-45) per 100,000 people and for adults it is 201 (95% CI, 197-204) per 100,000 people.1 However, a large-scale population-based study found that CD is becoming more common in Asia, especially in China (122/100,000) and Japan, and that the symptoms in patients with CD are often more severe in Asia than in the West.2,3,4
Penner and Crohn5 first reported that perianal fistulas were a complication of CD in 1938. Sometimes perianal fistula is the first recognizable symptom of CD. Perianal fistulas in CD may arise from inflamed or infected anal glands and/or penetration of fissures or ulcers in the rectum or anal canal. Some studies have shown that CD patients with perianal fistulas often require a combined surgical and medical approach because of frequent recurrence and abscess formation.6
Infliximab (IFX) and adalimumab (ADA) have proven to be effective in the treatment of perianal fistulas in CD,7,8 but it is not clear which is more effective. Therefore, the aim of this study was to determine the clinical efficacy of ADA compared with IFX in the treatment of perianal fistulas in CD, and to analyze the risk factors associated with recurrence or aggravation of the fistula tract.
All 47 consecutive CD patients treated for perianal fistulas with IFX from September 2005 to December 2010 (n=31) or with ADA from November 2010 to May 2012 (n=16) were enrolled in this study.
The International Organization for the Study of Inflammatory Bowel Disease (IOIBD) index, the CRP levels, and the number of recurrent or aggravated cases during the follow-up period were retrospectively analyzed. The IOIBD index consists of the following 10 factors: abdominal pain, diarrhea (>6 times per day), an anal lesion, a fistula, other complications, an abdominal tumor, weight loss, high fever, abdominal tenderness, and hemoglobin <10 g/dL. Each factor is scored as yes=1 point and no=0. All patients consented to receive either ADA or IFX treatment as part of a compassionate-use program. The patients were given details about each therapy and selected their own treatment. Clinical follow-up took place at 2, 6, and 8 weeks, and at 8-week intervals for 2 or more years after initial treatment.
The CD diagnosis was based on clinical symptoms, endoscopies, histopathological biopsies, CRP and other relevant blood examinations. The diagnosis of perianal fistula was based on the results of an MRI, endoanal ultrasound, and an examination under anesthesia by experienced colorectal surgeons. Fistulotomy was done for the SC fistula and a drainage seton was used for other fistulas. The indication for tumor necrosis factor-╬▒ (TNF-╬▒) antibodies was based on the abdominal condition. When steroid hormones or immunomodulatory agents showed a loss of response, TNF-╬▒ antibodies were considered. IFX (5 mg/kg) induction was administered at week 0, 2, and 6 and maintenance therapy at 8-week intervals. ADA induction treatment was started at a dose of 160 mg and an additional 80 mg 2 weeks later. Maintenance therapy was 40 mg every 2 weeks.
The following definitions were taken from the IOIBD guidelines: recurrence is the reappearance of symptoms after healing, and aggravation is defined as discharge, swelling, or pain that becomes more serious without treatment. Complete healing is defined as no symptoms or drainage for a minimum of 2 weeks.
Quantitative variables were analyzed using Student t-test. Categorical data are shown as the number and proportion of cases (%). The chi-square test was used to assess the categorical variables. Univariable and multivariable logistic regression were used to assess the risk factors for recurrence or aggravation. The OR for the outcomes (including 95% CI) was estimated for each variable in the model. Statistical analysis was performed by using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). A P-value less than 0.05 was considered statistically significant.
Twenty-seven of the 31 patients (87.1%) were male and four (12.9%) were female, with a mean age of 29.2┬▒8.9 years and a mean disease duration of 7.2┬▒6.8 years. According to the American Gastroenterological Association classification, 54.8% of the patients (17/31) had a simple anal fistula and 45.2% (14/31) had a complex anal fistula; 64.5% (20/31) required a seton. The follow-up period was 68.1┬▒11.7 months.
Fourteen of the 16 patients (87.5%) were male and two (12.5%) were female, with a mean CD onset age of 32.4┬▒9.7 years and a mean disease duration of 8.9┬▒6.5 years. The fistula classification were as follows: 56.3% of the patients (9/16) had a simple anal fistula and 43.8% (7/16) had a complex anal fistula; 68.8% (11/16) required a seton. The follow-up period was 36.1┬▒3.9 month.
There were no differences in gender, disease duration, age of CD onset, intestinal location (large intestine and/or small intestine), fistula classification, seton placement, IOIBD score, CRP, and frequency of injection between the IFX and ADA groups (P>0.05) (Table 1).
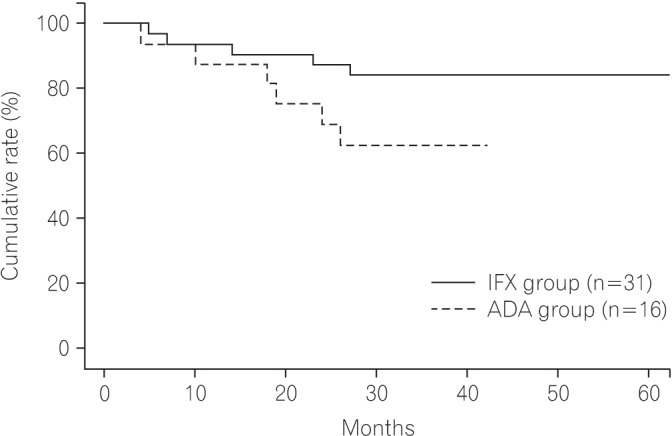
The cumulative rate of nonrecurrence or aggravation of fistula was 62.5% in the ADA group and 83.9% in the IFX group at 24 months after treatment (Fig. 1).
The risk factors for recurrence or aggravation were significantly correlated with seton placement in this study (P<0.05). Moreover, the risk factors may be related to gender and fistula classification but no clear correlation was found (P<0.05). Age, intestinal location, disease duration, IOIBD score, CRP level, elemental diet, immunomodulator use, follow-up period, and IFX/ADA induction were not identified as risk factors for recurrence or aggravation (P<0.05) (Table 2).
The risk factors for recurrence or aggravation were correlated with seton placement (P<0.05). Moreover, the risk factors may be related to fistula classification but no clear correlation was found (P<0.05). There was no significant correlation between the risk factors for recurrence or aggravation and IFX/ADA induction (P<0.05) (Table 3).
TNF-╬▒ antibodies (IFX, ADA, and certolizumab) are effective for both induction and maintenance in moderate to severe CD in adults (including patients with a perianal fistula).9 IFX is a chimerical monoclonal antibody comprised of 75% human and 25% mouse sequences. However, the murine component is potentially antigenic for humans. Repeated infusion may cause the patient's immune system to produce antibodies against IFX that may reduce the efficacy of the drug, and may even cause anaphylactic shock.10 In order to lower the risk of negative immunogenic responses induced by chimeric antibodies, new studies attempted to replace the mouse-derived sequences to create fully human monoclonal antibodies. The result was the development of ADA, the first fully human antibody to be approved for clinical treatment. Some studies have found that ADA was more effective in short-term clinical response/remission, in long-term remission, and in obtaining complete fistula healing, compared with placebo. ADA has also proven to be effective in patients who no longer respond to or who develop intolerance to IFX.11
Perianal fistula in CD is usually difficult to treat because of the high rate of relapse after cessation of treatment. Although the introduction of IFX has increased the efficacy, the results are still disappointing, especially for complex fistulas. A British study showed that only 29% and 42% of the patients achieved complete or partial remission, respectively, when treatment combined a surgical procedure with IFX.12 Echarri et al.13 found that complete cessation of drainage occurred in 50% of the short-term cases, and that 87.5% were able to maintain this effect over time (follow-up period of 48 weeks). Sands et al.14 reported abscess development in patients with fistulizing CD was not dependent on cumulative IFX exposure. The efficacy of ADA for perianal fistula with CD is still not very clear because a dedicated controlled study has not yet been done. Casta├▒o-Milla et al.8 reported retrospective data showing that ADA was effective for the treatment of perianal fistulas in CD patients naive to anti-TNF drugs. Our study examined the clinical efficacy of ADA versus IFX in the treatment of perianal fistulas in CD and the risk factors for fistula tract recurrence or aggravation were analyzed.
The retrospective nature of this study was a limiting factor. The treatment of the IFX group began 5 years earlier than that of the ADA group. Thus, the follow-up period was longer in the IFX group. We compared the efficacy of ADA and IFX and found that there were no differences in gender, disease duration, age of CD onset, intestinal location, fistula classification, seton placement, IOIBD score, CRP, and frequency of injection between the two groups.
The rate of nonrecurrence or aggravation of fistula was 62.5% in the ADA group and 83.9% in the IFX group, during a follow-up period of 24 months. We hypothesized that ADA was less effective than IFX for anal fistula in CD but found that there was no significant statistical difference between the two forms of treatment. However, we found that the seton was an independent risk factor for recurrence or aggravation of the fistula tract. Although this risk factor did not correlate with fistula classification, we hypothesized that a risk factor may be the fistula classification itself, because we only used a seton in patients with a complex fistula. Patients with a complex fistula were likely to experience recurrence or aggravation. The results were consistent with the findings of a study by Tozer et al.15 We also found that the risk factors may be gender specific because no female patients in our study experienced recurrence or aggravation. However, further research is needed to support these findings. The rates of loss of response were 12.9% and 25.0% in the IFX and ADA groups, respectively. When a biologic shows a loss of response and the patient experiences a recurrence of fistula, we reinsert a seton and use another biologic.
In conclusion, our results suggest that ADA may be less effective than IFX in the treatment of perianal fistula in CD. Fistula classification may also be an important risk factor, as complex fistulas are more likely to show relapse or aggravation.
References
1. Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn's disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol 2007;5:1424-1429.PMID: 17904915.


2. Ng SC, Tang W, Ching JY, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-Pacific Crohn's and colitis epidemiology study. Gastroenterology 2013;145:158-165.e2.PMID: 23583432.


3. Zeng Z, Zhu Z, Yang Y, et al. Incidence and clinical characteristics of inflammatory bowel disease in a developed region of Guangdong Province, China: a prospective population-based study. J Gastroenterol Hepatol 2013;28:1148-1153.PMID: 23432198.


4. Yao T, Matsui T, Hiwatashi N. Crohn's disease in Japan: diagnostic criteria and epidemiology. Dis Colon Rectum 2000;43(10 Suppl): S85-S93.PMID: 11052483.


5. Penner A, Crohn BB. Perianal fistulae as a complication of regional ileitis. Ann Surg 1938;108:867-873.PMID: 17857277.



6. Marzo M, Felice C, Pugliese D, et al. Management of perianal fistulas in Crohn's disease: an up-to-date review. World J Gastroenterol 2015;21:1394-1403.PMID: 25663759.



7. Hukkinen M, Pakarinen MP, Piekkala M, Koivusalo A, Rintala R, Kolho KL. Treatment of complex perianal fistulas with seton and infliximab in adolescents with Crohn's disease. J Crohns Colitis 2014;8:756-762.PMID: 24447625.



8. Casta├▒o-Milla C, Chaparro M, Saro C, et al. Effectiveness of adalimumab in perianal fistulas in Crohn's disease patients naive to anti-TNF therapy. J Clin Gastroenterol 2015;49:34-40.PMID: 25485513.


9. Kawalec P, Mikrut A, Wi┼øniewska N, Pilc A. Tumor necrosis factor-alpha antibodies (infliximab, adalimumab and certolizumab) in Crohn's disease: systematic review and meta-analysis. Arch Med Sci 2013;9:765-779.PMID: 24273556.



10. Su CG, Lichtenstein GR. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. Gastroenterology 2003;125:1544-1546.PMID: 14598275.


11. Ueno F, Matsui T, Matsumoto T, et al. Evidence-based clinical practice guidelines for Crohn's disease, integrated with formal consensus of experts in Japan. J Gastroenterol 2013;48:31-72.PMID: 23090001.


12. Antakia R, Shorthouse AJ, Robinson K, Lobo AJ. Combined modality treatment for complex fistulating perianal Crohn's disease. Colorectal Dis 2013;15:210-216.PMID: 22672653.


13. Echarri A, Castro J, Barreiro M, Carpio D, Pereira S, Lorenzo A. Evaluation of adalimumab therapy in multidisciplinary strategy for perianal Crohn's disease patients with infliximab failure. J Crohns Colitis 2010;4:654-660.PMID: 21122576.



14. Sands BE, Blank MA, Diamond RH, Barrett JP, Van Deventer SJ. Maintenance infliximab does not result in increased abscess development in fistulizing Crohn's disease: results from the ACCENT II study. Aliment Pharmacol Ther 2006;23:1127-1136.PMID: 16611273.


15. Tozer P, Ng SC, Siddiqui MR, et al. Long-term MRI-guided combined anti-TNF-alpha and thiopurine therapy for Crohn's perianal fistulas. Inflamm Bowel Dis 2012;18:1825-1834.PMID: 22223472.


Fig.┬Ā1
Cumulative rate of nonrecurrence or aggravation of fistula. IFX, infliximab; ADA, adalimumab.
Table┬Ā1
Patient Characteristics (n=47)
Table┬Ā2
Univariate Analysis of the Risk Factors for Recurrence or Aggravation
- TOOLS






