|
 |
- Search
| Intest Res > Volume 13(1); 2015 > Article |
|
Abstract
Background/Aims
Patients with ulcerative colitis (UC) are at high risk for cytomegalovirus (CMV) reactivation. The usefulness of the CMV antigenemia assay in active UC patients has rarely been studied. We assessed whether the assay detects CMV colitis and predicts clinical outcomes in patients with UC.
Methods
We retrospectively reviewed the medical records of patients hospitalized for moderate-to-severe UC from 2003 to 2012. Positive CMV antigenemia was defined as ā„1 pp65-positive cell per 2Ć105 polymorphonuclear neutrophils. CMV colitis was defined as the presence of inclusion bodies and/or positive immunohistochemistry in the colonic mucosa. The primary outcome was steroid refractoriness, defined as the absence of clinical improvement after intravenous high-dose steroid administration.
Results
A total of 43 patients were enrolled. CMV antigenemia was detected in 12 (27.9%) patients. Positive CMV antigenemia was significantly associated with CMV colitis (P =0.001). The sensitivity and specificity of positive CMV antigenemia for diagnosing CMV colitis were 66.7% and 87.1%, respectively. Steroid refractoriness was found in 11 of 12 (91.7%) and 12 of 31 (38.7%) patients with positive and negative CMV antigenemia, respectively (P =0.002). The independent predictors for steroid refractoriness were positive CMV antigenemia (adjusted odds ratio [OR], 7.73; 95% confidence interval [CI], 1.22-49.19; P =0.030) and a shorter duration from the diagnosis of UC (adjusted OR, 0.99; 95% CI, 0.98-0.99; P =0.025).
Human cytomegalovirus (CMV) infection is common, and the prevalence of CMV infection has been estimated to range from 30% to 100% worldwide, depending on the age of the populations tested.1 CMV reactivation usually occurs as an opportunistic infection and leads to morbidity or mortality in immunocompromised individuals.2 Patients with UC are also at high risk for CMV reactivation because they are frequently treated with immunosuppressive drugs.3 Although whether CMV is a primary aggravating cause or by-product in the exacerbation of UC remains to be elucidated, antiviral therapy has allowed some patients who have not responded to conventional UC treatment to avoid colectomy.4 The current guideline recommends that CMV colitis should be excluded in immunomodulator refractory cases of IBD before increasing immunomodulator therapy.5 Therefore, the presence of CMV colitis should be confirmed in patients with an exacerbation of UC, especially refractory colitis.
The CMV antigenemia assay detects the CMV pp65 antigen in circulating polymorphonuclear neutrophils (PMNs) in the blood by staining with fluorescent antibodies that are specific for pp65 antigens.4 Previous studies have shown that this semiquantitative technique is useful for diagnosing and monitoring CMV infection in immunocompromised patients, such as bone marrow6 and solid organ (e.g., kidney and liver) transplant recipients,7,8,9 or those with acquired immunodeficiency syndrome (AIDS).10,11 Furthermore, CMV antigenemia-guided pre-emptive ganciclovir therapy was effective in patients who received allogenic hematopoietic stem cell transplantation.12 However, the clinical usefulness of the CMV antigenemia assay in patients with UC has rarely been studied. The aim of this study was to assess whether the CMV antigenemia assay detects CMV colitis and predicts clinical outcomes in patients with moderate-to-severe UC.
We retrospectively reviewed the medical records of moderate-to-severe UC patients aged >18 years who were hospitalized due to an acute exacerbation at Seoul National University Hospital from July 2003 to June 2012. Those patients who underwent the CMV antigenemia assay at the time of admission were enrolled. Based on each physician's decision, the CMV antigenemia assay was performed to evaluate CMV reactivation. The diagnosis of UC was based on clinical, endoscopic, radiologic, and histologic findings. UC severity was assessed using the Mayo Clinic scoring system.13 All patients had Mayo Clinic scores (disease activity index) between 6 and 12 and underwent a colonoscopy or sigmoidoscopy at the time of admission. The initial evaluation for CMV colitis was performed using the CMV antigenemia assay and endoscopic and histopathologic examination. Patients who had previously undergone solid organ transplantation were excluded.
Generally, patients were treated with an intravenous high-dose corticosteroid (300-400 mg/day hydrocortisone) for 7 to 14 days until clinical improvement was observed. As defined by the Mayo Clinic scoring system, clinical improvement was considered a greater than 2-point decline in the Mayo Clinic score compared with the initial score. Steroid refractoriness was defined as the absence of clinical improvement after a 7- to 14-day course of intravenous high-dose steroids, regardless of the CMV infection status.14,15 After the steroid therapy, a repeat colonoscopy or sigmoidoscopy was performed with reevaluation for concomitant CMV colitis and Clostridium difficile infection, especially in patients with steroid refractoriness. A stool culture was also performed for diagnosis of C. Difficile infection in patients with steroid refractoriness. Corticosteroids were continued and properly tapered in patients who responded to the high-dose regime. In patients with steroid refractoriness and concomitant CMV colitis, intravenous ganciclovir was administered at 5 mg/kg every 12 hours for 2 weeks. In patients with steroid refractoriness and without CMV colitis, rescue therapy with cyclosporine, infliximab, empirical ganciclovir, or a colectomy was performed at the physician's discretion. The primary clinical outcome was steroid refractoriness. The secondary clinical outcome was the need for colectomy within 2 months due to failure of the medical treatment. The institutional review board at Seoul National University Hospital approved this study.
All of the endoscopic images were digitalized and retrospectively reviewed by a single, experienced endoscopist who was blinded to the patient information. The endoscopic severity of UC was assessed using the Matts grading system. 16 The endoscopic severity of UC was scored as follows: normal=1, mild granularity and edema=2, marked granularity, edema, and spontaneous bleeding=3, and severe ulceration= 4. Left-sided colitis referred to inflammation extending up to, but not beyond, the splenic flexure. Extensive colitis was defined as disease extending beyond the splenic flexure.
The CMV antigenemia assays were performed using ethylenediaminetetraacetic acid-treated whole blood samples, according to previously reported methods.17 In short, the blood samples were fractionated by dextran sedimentation and erythrocyte lysis. A cytospin slide was prepared after centrifugation of the blood samples, fixed with formaldehyde, sequentially immunostained with a monoclonal antibody (C10/C11) against the pp65 antigen (Clonab CMV; Biotest, Dreieich, Germany), and reacted with fluorescein isothiocyanate-conjugated goat anti-mouse IgG antibody (Clonab Ig-fluorescein isothiocyanate; Biotest, Dreieich, Germany). The results were expressed as the number of CMV pp65-positive cells per slide, with each slide containing 2Ć105 PMNs. Positive CMV antigenemia was defined as at least 1 pp65-positive cell per 2Ć105 PMNs.
An initial colonoscopy or sigmoidoscopy was performed to assess the severity of UC and the presence of concomitant CMV colitis. If ulcers were present, multiple biopsies were obtained at the margin and base of the largest ulcer for histologic examination. If no ulcer was detected, biopsies were obtained from the areas with the most severe inflammation. Colonic biopsy specimens were evaluated with H&E staining and immunohistochemical (IHC) staining using monoclonal antibodies against CMV. CMV colitis was defined as the presence of at least one inclusion body in the H&E-stained sections and/or positive IHC staining for CMV in the colonic mucosa from the initial endoscopy.15,18
Statistical analysis was performed with SPSS (version 17.0; Chicago, IL, USA). Pearson's chi-squared test, Fisher's exact test, and Student's t-test were used, as appropriate, to calculate the statistical significance of the demographic and clinical variables. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for the ability of the positive CMV antigenemia assay to predict CMV colitis using 2Ć2 tables. The receiver operating characteristic curve was used to determine the cut-off value of the CMV antigenemia assay for detecting CMV colitis. Multivariate analysis with binary logistic regression models was used to identify risk factors for steroid refractoriness. P-values <0.05 were considered statistically significant.
We retrospectively reviewed the medical records of 146 moderate-to-severe UC patients aged >18 years who were hospitalized due to an acute exacerbation from July 2003 to June 2012. Of those, 46 patients in whom the CMV antigenemia assay was performed at the time of hospitalization were included. Among them, 3 were excluded due to a past medical history of solid organ transplantation (2 patients) or due to insufficient information in the medical records (1 patient). Finally, 43 patients with moderate-to-severe active UC were enrolled in the study. The study population comprised 20 males (46.5%), and the mean age was 49.2Ā±16.1 years (range, 24-92). There were 25 (58.1%) and 18 (41.9%) patients with left-sided colitis and extensive colitis, respectively. The mean initial disease activity index was 10.1Ā±1.7, and the UC was severe in 20 (46.5%) patients according to the Mayo Clinic scoring system. The mean Matts grade was 3.6Ā±0.6. In addition, 29 (67.4%) patients had been treated with corticosteroids, 3 (7.0%) with azathioprine, and 3 (7.0%) with cyclosporine prior to hospitalization. CMV antigenemia was detected in 12 (27.9%) patients. There were no significant differences in terms of the baseline characteristics between the positive and negative CMV antigenemia groups, except for the extent of colitis (P =0.006) and the endoscopic severity measured by the Matts grading system (P =0.042) (Table 1).
CMV colitis was diagnosed in 8 (66.7%) of 12 patients positive for CMV antigenemia in H&E-stained slides and/or positive IHC staining for CMV. In contrast, 4 (12.9%) of 31 patients negative for CMV antigenemia had CMV colitis. Positive CMV antigenemia was significantly associated with CMV colitis (P =0.001).
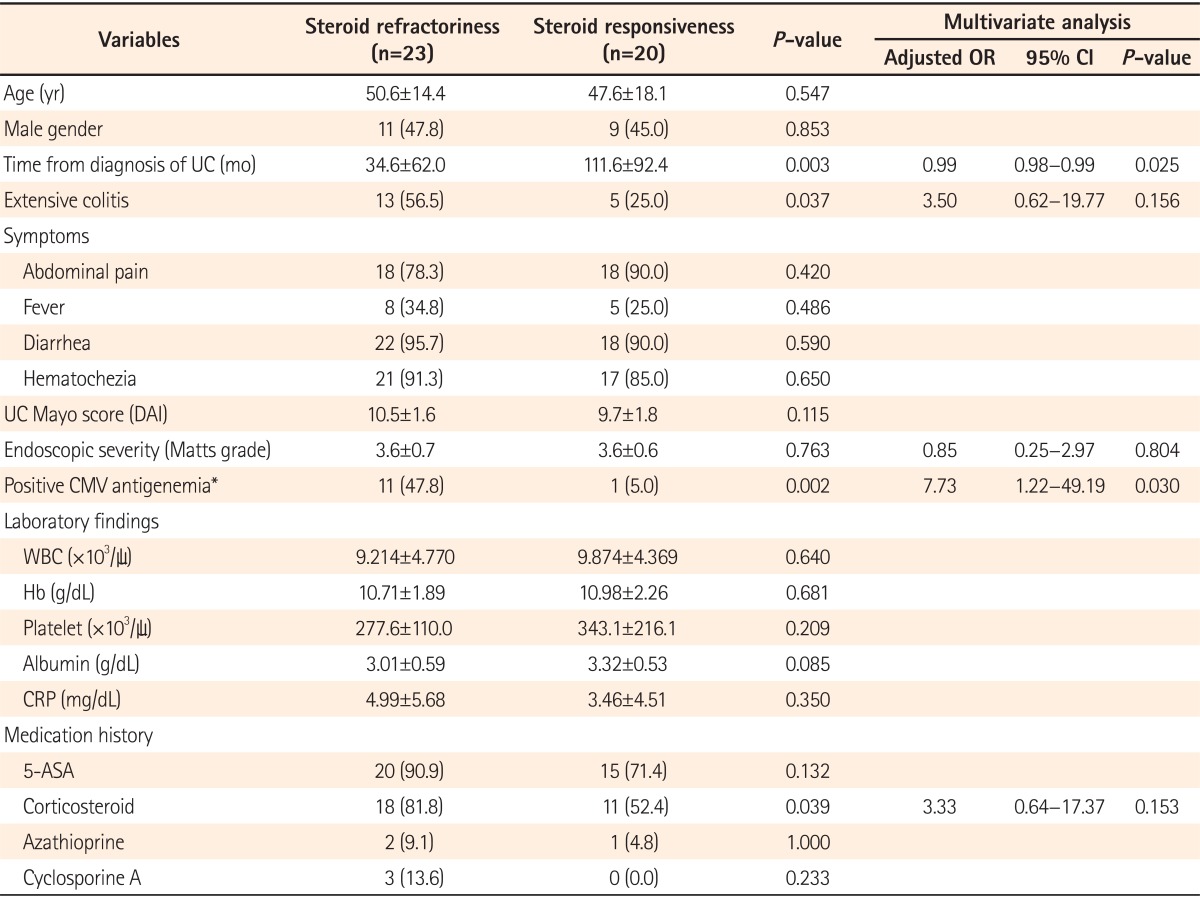
The sensitivity, specificity, PPV, and NPV of a positive CMV antigenemia assay (defined as ā„1 pp65-positive cell per 2Ć105 PMNs) for diagnosing CMV colitis were 66.7% (95% CI, 53.1-80.3), 87.1% (95% CI, 81.1-93.1), 66.7% (95% CI, 53.1-80.3), and 87.1% (95% CI, 81.1-93.1), respectively. The receiver operating characteristic curve for the CMV antigenemia assay is shown in Fig. 1. The optimal cut-off value of the CMV antigenemia assay was 2 pp65-positive cells per 2Ć105 PMNs (sensitivity, 66.7% [95% CI, 53.1-80.3]; specificity, 90.3% [95% CI, 85.0-95.6]).
The clinical characteristics and outcomes of the 12 patients positive for CMV antigenemia are shown in Table 2. The titers of the CMV antigenemia assay ranged from 1 to 11 pp65-positive cells per 2Ć105 PMNs. 9 (75.0%) of these patients had extensive colitis. According to the Mayo Clinic scoring system, UC was severe in 7 (58.3%) patients. 1 (8.3%) patient responded to high-dose steroid therapy (No. 7 in Table 2), and the remaining 11 (91.7%) had steroidrefractory UC. Of the 11 patients with steroid refractoriness, 8 (72.7%) were treated with ganciclovir due to concomitant CMV colitis (No. 1, 4-6, and 8-11 in Table 2). 6 (75.0%) of the 8 patients with steroid refractoriness and concomitant CMV colitis went into remission after ganciclovir therapy for 2 weeks (No. 1, 6, and 8-11 in Table 2). Of the 3 patients with steroid refractoriness without CMV colitis, one improved after rescue therapy with cyclosporine (No. 2 in Table 2), and the other 2 received a colectomy within 2 months (No. 3 and 12 in Table 2). The quantitative titer of the CMV antigenemia assay was not associated with the extent and severity of UC in the patients positive for CMV antigenemia. Moreover, the quantitative titer of the assay was not associated with steroid refractoriness and the colectomy requirement in the patients positive for CMV antigenemia.
The clinical course in all 43 patients is shown in Fig. 2. Overall, 23 (53.5%) patients had steroid-refractory UC, and 11 (25.6%) received a colectomy within 2 months. Among 12 steroid-refractory UC patients without initially concomitant CMV colitis, none had complicated CMV and C. difficile infection after high-dose steroid therapy. Of the 31 patients negative for CMV antigenemia, steroid refractoriness was found in 12 (38.7%) patients. Of these, 3 (25.0%) patients with steroid refractoriness and concomitant CMV colitis received ganciclovir therapy for 2 weeks. The clinical outcomes were improved after ganciclovir alone in 1 patient, after rescue therapy with infliximab in 1 patient, and after colectomy in 1 patient. Of the 9 steroid-refractory UC patients negative for CMV antigenemia without CMV colitis, 4 (44.4%) went into remission after empirical ganciclovir (1 patient) or cyclosporine therapy (3 patients), and the remaining 5 (55.6%) underwent a colectomy within 2 months. 1 (5.3%) of the 19 steroid-responsive UC patients negative for CMV antigenemia underwent a delayed colectomy for complicated colonic perforation.
Positive CMV antigenemia was strongly associated with steroid refractoriness (P =0.002). The titer of the CMV antigenemia assay showed a tendency to be higher in the patients with steroid-refractory UC (2.1Ā±2.7 cells/2Ć105 PMNs) than in those with steroid-responsive UC (0.7Ā±2.5 cells/2Ć105 PMNs) (P=0.058). In addition, CMV colitis was significantly more common in patients with steroid-refractory UC (45.5% [10 of 22]) than in those with steroid-responsive UC (9.5% [2 of 21]) (P =0.009). However, no significant difference was found in the colectomy rate within 2 months between the positive (33.3% [4 of 12]) and negative CMV antigenemia groups (22.6% [7 of 31]) (P =0.467) (Table 3).
The predictive factors for steroid refractoriness under univariate analyses were positive CMV antigenemia (P =0.002), a shorter duration from the diagnosis of UC (P =0.003), extensive colitis (P =0.037) and previous oral corticosteroid administration (P =0.039). Multivariate analysis indicated that steroid refractoriness was significantly more common in patients positive for CMV antigenemia (adjusted OR, 7.73; 95% CI, 1.22-49.19; P =0.030) and in those with a shorter duration from the diagnosis of UC (adjusted OR, 0.99; 95% CI, 0.98-0.99; P =0.025) (Table 4).
We determined that positive CMV antigenemia was an independent predictor of steroid refractoriness in patients with moderate-to-severe UC. Although systemic corticosteroids remain the standard treatment for patients with an exacerbation of UC, approximately 27% of patients require an early colectomy after corticosteroids.19 Rescue therapies, including cyclosporine or infliximab, have been shown to be effective in preventing the requirement for an early colectomy. 20,21,22 Thus, the identification of early predictors of steroid-refractory UC would avoid the ineffective continuation of corticosteroids, and facilitate a more timely initiation second-line rescue therapy.
In this study, both positive CMV antigenemia and CMV colitis were significantly common in patients with steroid-refractory UC. In accordance with our results, previous reports have shown that severe refractory colitis is associated with documented CMV colitis in patients with an exacerbation of UC.23 Moreover, many retrospective studies have reported that 82% of UC patients with a CMV infection are non-responders to corticosteroids, suggesting that CMV colitis is a probable cause of steroid-refractory UC.24
In contrast, a previous study demonstrated that CMV reactivation did not affect the rates of remission and colectomy in patients with moderate-to-severe UC.25 In the study, however, the rate of early rescue therapy with cyclosporine was significantly higher in UC patients with CMV reactivation than in those without CMV reactivation, which could improve the rate of remission in those with CMV reactivation.25 Therefore, these findings support the hypothesis that early rescue therapy in patients with an exacerbation of UC and concomitant CMV reactivation reduces the requirement for colectomy.
In this study, 7 (63.6%) of the 11 patients with steroid refractoriness improved following rescue therapy with ganciclovir (6 patients) or cyclosporine (1 patient), and there was no association between the detection of CMV antigenemia and need for colectomy. The detection of CMV antigenemia, which is indicative of CMV reactivation, can be a good predictor that allows for the early detection of steroid-refractory UC and indicates the need for rescue therapy. Because the CMV antigenemia assay has a short processing time (less than 6 hours) and does not require a specialized laboratory, 26 it is expected to be a clinically useful method for the early prediction of steroid-refractory UC.
We showed that 75% of steroid-refractory UC patients positive for CMV antigenemia with concomitant CMV colitis went into remission after ganciclovir treatment. Kim et al.15 reported that ganciclovir might be the treatment of choice in patients with steroid-refractory UC and concomitant CMV infection. In contrast, Matsuoka et al.25 demonstrated that 72% of patients with CMV reactivation entered remission without antiviral therapy after conventional immunosuppressant therapy, but that none showed any evidence of CMV colitis in their colonic tissue. In an earlier report, 5 of 6 (83.3%) patients with UC and concomitant CMV colitis responded to ganciclovir,27 a finding that is comparable with our results. These findings suggest that antiviral therapy might be beneficial for patients with steroid-refractory UC and concomitant CMV colitis. Because positive CMV antigenemia showed a high risk of steroid refractoriness, moderate-to-severe UC patients positive for CMV antigenemia should be carefully evaluated for concomitant CMV colitis using endoscopic and histologic examinations. Additionally, early ganciclovir treatment might be considered as a rescue therapy in patients positive for CMV antigenemia with concomitant CMV colitis.
The CMV antigenemia assay is a widely used method for detecting CMV reactivation in a variety of clinical settings.28 In general, the detection of CMV pp65-positive cells has a sensitivity of 60-100% and a specificity of 83-100% for CMV reactivation.29 However, few studies have examined the diagnostic value for CMV colitis in UC patients. In the present study, the sensitivity and specificity of the positive CMV antigenemia assay for diagnosing CMV colitis in patients with moderate-to-severe UC were 66.7% and 87.1%, respectively. These results are in agreement with those of a recent study showing the diagnostic performance of the CMV antigenemia assay in UC patients.30
In addition, an earlier study reported that the sensitivity and specificity of the CMV antigenemia assay for CMV gastrointestinal disease were 54% and 88% respectively in 121 patients with secondary immunodeficiency diseases.31 In 99 immunocompromised patients, none of who had IBD, the sensitivity and specificity of the CMV antigenemia assay for CMV gastrointestinal disease were 65.4% and 93.6%, respectively.32 Our results in UC patients were comparable with those reported by earlier studies conducted in populations with other immunodeficiency diseases. These findings suggest that the CMV antigenemia assay has low sensitivity but high specificity for detecting CMV colitis in immunocompromised patients regardless of the underlying disease status.
We also determined that the cut-off level of 2 pp65-positive cells per 2Ć105 PMNs showed the highest sensitivity and specificity for diagnosing CMV colitis. However, this threshold might be of limited value due to the minimal difference in the specificity between the cut-off values of one and 2 pp65-positive cells.
We showed that the maximum level measured in the CMV antigenemia assay in patients with UC was 11 pp65-positive cells per 2Ć105 PMNs. Our results are in agreement with those of a prospective study in which the titer of the CMV antigenemia assay was 1 pp65-positive cell in 47.1% of UC patients positive for CMV antigenemia, and only 1 (5.9%) patient showed more than 10 positive cells.25 In fact, the ranges of the CMV antigenemia assay vary among patient settings. In a previous report involving patients who underwent autologous marrow or peripheral blood stem cell transplantation, the maximum level of the assay was 135 pp65-positive cells.33 In other reports, a cut-off level of 25 pp65-positive cells showed the highest PPV for symptomatic CMV infection in renal transplant recipients.34,35 Therefore, patients with an exacerbation of UC have a relatively low viral load in their blood because they were treated with less intensive immunosuppressive agents than those used for bone marrow or solid organ transplant recipients.
We also demonstrated that a shorter duration of disease was an independent predictor for steroid refractoriness in active UC patients. Some studies have demonstrated that a shorter duration of disease predicts therapeutic failure in active UC patients treated with intravenous corticosteroids,30,36 while others found no association between duration of disease and the response to systemic corticosteroids.37,38 Recently, Garcia-Planella et al.39 demonstrated that the earlier systemic corticosteroids were introduced in the course of UC, the higher the risk of relapse and corticosteroids reintroduction was. It suggests that the sooner systemic corticosteroids are required from the time of UC diagnosis, the poorer is the prognosis. Therefore, close monitoring followed by rescue therapy might be considered for active UC patients who required intravenous high dose corticosteroids in the early course of disease.
Our study has several limitations due to its retrospective nature.
First, selection bias might occur because only hospitalized patients who were tested for CMV antigenemia were included. Because the CMV antigenemia assay was performed based on each physician's decision, it is possible that more patients with concomitant CMV colitis and steroid-refractory UC were included in this study. Moreover, the patients who were enrolled in the tertiary care hospital-based study may be biased toward those with more severe colitis and poorer prognosis than the cases in population-based studies.
Second, the sample size was small. We showed that symptom severity, laboratory parameters such as CRP or serum albumin, disease extent, and endoscopic severity could not predict lack of response to systemic corticosteroids in patients with an exacerbation of UC. Although many studies reported an extreme variety in results regarding the predictive factors for steroid refractoriness in patients with an exacerbation of UC,19 a significant difference might not have been confirmed among independent predictors due to the selection bias or small sample size in our study.
Third, a follow-up CMV antigenemia assay was not routinely carried out. Among eight steroid-refractory UC patients positive for CMV antigenemia, seroconversion was confirmed by a follow-up CMV antigenemia assay after ganciclovir treatment in three patients who showed improvement. In contrast, it was unclear whether seroconversion could occur in the other 5 patients because they did not undergo the follow-up testing.
Fourth, colonic tissue PCR for CMV DNA was not performed for diagnosing CMV colitis. The guideline recommends that CMV colitis should be evaluated, preferably by tissue PCR or IHC, in refractory UC patients.5 Moreover, a previous study showed that CMV DNA load in inflamed colonic tissue predicts the resistance to intravenous steroids in patients with exacerbation of UC.40 This suggests that the detection of CMV DNA in colon tissue without histological features of CMV colitis may represent low-level reactivation of CMV.
Finally PCR detection of CMV DNA in blood was not conducted in this study, and the titers of the CMV antigenemia assays could not be compared to blood CMV DNA levels.
In conclusion, the CMV antigenemia assay has low sensitivity but high specificity for detecting CMV colitis, and may predict steroid refractoriness in patients with moderate-to-severe UC. We suggest that early rescue therapy with cyclosporine or infliximab should be considered in moderate-to-severe UC patients with CMV antigenemia.
References
1. Krech U. Complement-fixing antibodies against cytomegalovirus in different parts of the world. Bull World Health Organ 1973;49:103-106.PMID: 4363395.


2. Goodgame RW. Gastrointestinal cytomegalovirus disease. Ann Intern Med 1993;119:924-935.PMID: 8215005.


3. D'Ovidio V, Vernia P, Gentile G, et al. Cytomegalovirus infection in inflammatory bowel disease patients undergoing anti-TNFalpha therapy. J Clin Virol 2008;43:180-183.PMID: 18614396.


4. Lawlor G, Moss AC. Cytomegalovirus in inflammatory bowel disease: pathogen or innocent bystander? Inflamm Bowel Dis 2010;16:1620-1627.PMID: 20232408.


5. Rahier JF, Ben-Horin S, Chowers Y, et al. European evidencebased Consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis 2009;3:47-91.PMID: 21172250.



6. Gondo H, Minematsu T, Harada M, et al. Cytomegalovirus (CMV) antigenaemia for rapid diagnosis and monitoring of CMV-associated disease after bone marrow transplantation. Br J Haematol 1994;86:130-137.PMID: 8011521.


7. van den Berg AP, van der Bij W, van Son WJ, et al. Cytomegalovirus antigenemia as a useful marker of symptomatic cytomegalovirus infection after renal transplantation--a report of 130 consecutive patients. Transplantation 1989;48:991-995.PMID: 2556817.


8. van den Berg AP, Tegzess AM, Scholten-Sampson A, et al. Monitoring antigenemia is useful in guiding treatment of severe cytomegalovirus disease after organ transplantation. Transpl Int 1992;5:101-106.PMID: 1320889.


9. van den Berg AP, Klompmaker IJ, Haagsma EB, et al. Antigenemia in the diagnosis and monitoring of active cytomegalovirus infection after liver transplantation. J Infect Dis 1991;164:265-270.PMID: 1649873.



10. Francisci D, Tosti A, Preziosi R, Baldelli F, Stagni G, Pauluzzi S. Role of antigenemia assay in the early diagnosis and prediction of human cytomegalovirus organ involvement in AIDS patients. Eur J Clin Microbiol Infect Dis 1995;14:498-503.PMID: 7588822.


11. Reynes J, Montes B, Atoui N, Segondy M. Significance of cytomegalovirus (CMV)-pp65 antigenemia in the diagnosis of CMV disease in human immunodeficiency virus-infected patients. J Med Virol 1996;49:195-198.PMID: 8818964.


12. Kanda Y, Mineishi S, Saito T, et al. Pre-emptive therapy against cytomegalovirus (CMV) disease guided by CMV antigenemia assay after allogeneic hematopoietic stem cell transplantation: a single-center experience in Japan. Bone Marrow Transplant 2001;27:437-444.PMID: 11313674.



13. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987;317:1625-1629.PMID: 3317057.


14. Creed TJ, Probert CS, Norman MN, et al. Basiliximab for the treatment of steroid-resistant ulcerative colitis: further experience in moderate and severe disease. Aliment Pharmacol Ther 2006;23:1435-1442.PMID: 16669958.


15. Kim YS, Kim YH, Kim JS, et al. The prevalence and efficacy of ganciclovir on steroid-refractory ulcerative colitis with cytomegalovirus infection: a prospective multicenter study. J Clin Gastroenterol 2012;46:51-56.PMID: 21552140.


16. Matts SG. The value of rectal biopsy in the diagnosis of ulcerative colitis. Q J Med 1961;30:393-407.PMID: 14471445.

17. Ho SK, Lo CY, Cheng IK, Chan TM. Rapid cytomegalovirus pp65 antigenemia assay by direct erythrocyte lysis and immunofluorescence staining. J Clin Microbiol 1998;36:638-640.PMID: 9508287.



18. Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 2002;34:1094-1097.PMID: 11914998.



19. Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol 2007;5:103-110.PMID: 17142106.


20. Kohn A, Daperno M, Armuzzi A, et al. Infliximab in severe ulcerative colitis: short-term results of different infusion regimens and long-term follow-up. Aliment Pharmacol Ther 2007;26:747-756.PMID: 17697208.


21. Jarnerot G, Hertervig E, Friis-Liby I, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology 2005;128:1805-1811.PMID: 15940615.


22. Rolny P, Vatn M. Cyclosporine in patients with severe steroid refractory ulcerative colitis in the era of infliximab. Review article. Scand J Gastroenterol 2013;48:131-135.PMID: 23110487.


23. Kandiel A, Lashner B. Cytomegalovirus colitis complicating inflammatory bowel disease. Am J Gastroenterol 2006;101:2857-2865.PMID: 17026558.


24. Xue M, Chen SJ, Wang LJ, Du Y, Si JM. Cytomegalovirus: a probable cause of steroid-refractory ulcerative colitis. J Dig Dis 2013;14:160-165.PMID: 23324050.


25. Matsuoka K, Iwao Y, Mori T, et al. Cytomegalovirus is frequently reactivated and disappears without antiviral agents in ulcerative colitis patients. Am J Gastroenterol 2007;102:331-337.PMID: 17156136.


26. Boeckh M, Boivin G. Quantitation of cytomegalovirus: methodologic aspects and clinical applications. Clin Microbiol Rev 1998;11:533-554.PMID: 9665982.



27. Vega R, Bertran X, Menacho M, et al. Cytomegalovirus infection in patients with inflammatory bowel disease. Am J Gastroenterol 1999;94:1053-1056.PMID: 10201482.


28. Mori T, Mori S, Kanda Y, et al. Clinical significance of cytomegalovirus (CMV) antigenemia in the prediction and diagnosis of CMV gastrointestinal disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2004;33:431-434.PMID: 14676775.



29. Nakase H, Honzawa Y, Toyonaga T, et al. Diagnosis and treatment of ulcerative colitis with cytomegalovirus infection: importance of controlling mucosal inflammation to prevent cytomegalovirus reactivation. Intest Res 2014;12:5-11.PMID: 25349558.



30. Kim JW, Boo SJ, Ye BD, et al. Clinical utility of cytomegalovirus antigenemia assay and blood cytomegalovirus DNA PCR for cytomegaloviral colitis patients with moderate to severe ulcerative colitis. J Crohns Colitis 2014;8:693-701.PMID: 24405983.



31. Jang EY, Park SY, Lee EJ, et al. Diagnostic performance of the cytomegalovirus (CMV) antigenemia assay in patients with CMV gastrointestinal disease. Clin Infect Dis 2009;48:e121-e124.PMID: 19441977.



32. Nagata N, Kobayakawa M, Shimbo T, et al. Diagnostic value of antigenemia assay for cytomegalovirus gastrointestinal disease in immunocompromised patients. World J Gastroenterol 2011;17:1185-1191.PMID: 21448424.



33. Boeckh M, Stevens-Ayers T, Bowden RA. Cytomegalovirus pp65 antigenemia after autologous marrow and peripheral blood stem cell transplantation. J Infect Dis 1996;174:907-912.PMID: 8896489.



34. Kim CK, Song JH, Kim SM, et al. Clinical usefulness of human cytomegalovirus antigenemia assay after kidney transplantation. Transplantation 2003;75:2151-2155.PMID: 12829929.


35. Jung GO, Kim SJ, Choi GS, et al. The effect of cytomegalovirus antigenemia titer on the efficacy of preemptive therapy for the prevention of cytomegalovirus disease after kidney transplantation. Transplant Proc 2010;42:804-810.PMID: 20430177.


36. Meyers S, Lerer PK, Feuer EJ, Johnson JW, Janowitz HD. Predicting the outcome of corticoid therapy for acute ulcerative colitis. Results of a prospective, randomized, double-blind clinical trial. J Clin Gastroenterol 1987;9:50-54.PMID: 3031150.


37. Chakravarty BJ. Predictors and the rate of medical treatment failure in ulcerative colitis. Am J Gastroenterol 1993;88:852-855.PMID: 8503379.

38. Jeon HH, Lee HJ, Jang HW, et al. Clinical outcomes and predictive factors in oral corticosteroid-refractory active ulcerative colitis. World J Gastroenterol 2013;19:265-273.PMID: 23345950.



39. Garcia-Planella E, Manosa M, Van Domselaar M, et al. Longterm outcome of ulcerative colitis in patients who achieve clinical remission with a first course of corticosteroids. Dig Liver Dis 2012;44:206-210.PMID: 22079262.


40. Roblin X, Pillet S, Oussalah A, et al. Cytomegalovirus load in inflamed intestinal tissue is predictive of resistance to immunosuppressive therapy in ulcerative colitis. Am J Gastroenterol 2011;106:2001-2008.PMID: 21788989.



Fig.Ā 1
Receiver operating characteristic curve analysis of the cytomegalovirus (CMV) antigenemia assay for detecting CMV colitis. *CMV pp65-positive cells per 2Ć105 leukocytes. AUC, area under the curve.
Fig.Ā 2
Clinical course in patients with moderate-to-severe UC. CMV Ag, cytomegalovirus antigenemia.

TableĀ 1
Baseline Characteristics of Subjects

Values are presented as meanĀ±SD or n (%).
*Positive CMV Ag was defined as at least 1 CMV pp65-positive cell per 2Ć105 leukocytes.
ā Median (range).
CMV Ag, cytomegalovirus antigenemia; DAI, disease activity index; WBC, white blood cells; Hb, hemoglobin; 5-ASA, 5-aminosalicylic acid; IV, intravenous.
TableĀ 2
Clinical Characteristics and Outcomes in Patients Positive for Cytomegalovirus Antigenemia (CMV Ag)