 |
 |
- Search
| Intest Res > Volume 12(1); 2014 > Article |
|
Abstract
Background/Aims
Combination therapy utilizing tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) in conjunction with other anticancer agents, is a promising strategy to overcome TRAIL resistance in malignant cells. Recently, parthenolide (PT) has proved to be a promising anticancer agent, and several studies have explored its use in combination therapy. Here, we investigated the molecular mechanisms by which PT sensitizes colorectal cancer (CRC) cells to TRAIL-induced apoptosis.
Methods
HT-29 cells (TRAIL-resistant) were treated with PT and/or TRAIL for 24 hours. The inhibitory effect on proliferation was detected using the 3-(4, 5-dimethylthiazol-2yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Annexin V staining, cell cycle analysis, and Hoechst 33258 staining were used to assess apoptotic cell death. Activation of an apoptotic pathway was confirmed by Western blot.
Results
Treatment with TRAIL alone inhibited the proliferation of HCT 116 cells in a dose-dependent manner, whereas proliferation was not affected in HT-29 cells. Combination PT and TRAIL treatment significantly inhibited cell growth and induced apoptosis of HT-29 cells. We observed that the synergistic effect was associated with misregulation of B-cell lymphoma 2 (Bcl-2) family members, release of cytochrome C to the cytosol, activation of caspases, and increased levels of p53.
The tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is a member of the tumor necrosis factor family of cytokines. The binding of TRAIL to its receptors, death receptor 4 (DR4) and DR5, triggers apoptotic signaling. The activation of DR4 or DR5 recruits the Fas-associated death domain protein (FADD) and procaspase-8 to form the death-inducing signaling complex (DISC), which leads to the activation of the caspase cascade.1,2 Caspase activation can be suppressed by the inhibitor of apoptosis protein (IAP) family members, as well as by anti-apoptotic B-cell lymphoma 2 (Bcl-2) family proteins.3 TRAIL has been reported to trigger selective and specific apoptotic cell death events in vitro and in vivo, with no significant side effect on normal cells.2,4 The use of TRAIL in such therapy, is currently undergoing intensive clinical evaluation. Although TRAIL is a potent anti-tumor agent in preclinical models, it has also been reported that some tumor cells intrinsically process or acquire resistance to TRAIL. TRAIL resistance can occur at several steps in the signaling cascade and can arise through different mechanisms. In colorectal cancer (CRC) cells, several mechanisms contributing to TRAIL resistance have been reported, including deficient receptor redistribution to the membrane,5 mutation of caspase-8,6 cellular fas-associated death domain-like interleukin-1-╬▓-converting enzyme-inhibitory protein (cFLIP) expression,7 Bax deficiency,8 or through X-linked inhibitor of apoptosis protein (XIAP) expression.9 As a result, it is not recommended to use TRAIL as a single agent. Combined treatment with chemotherapeutic drugs has been shown to overcome TRAIL resistance in many cancer cell types.10-12
Parthenolide (PT), a naturally occurring agent, has been used for the treatment of fever and inflammatory disease.13 Over the past two decades, it has been established that PT induces apoptosis in various cancer cell types, including human hepatocellular carcinoma cells, human lung and stomach cancer cells, and glioblastoma cells.10,14-17 It has been shown that the apoptotic effect of PT is associated with the inhibition of nuclear factor ╬║B (NF-╬║B),18 and signal transducer and activator of transcription 3 (STAT3),18 enhanced oxidative stress,19 and activation of the mitochondria-mediated pathway.20 In our previous study, we found that PT effectively induced apoptosis in CRC cells by causing mitochondrial dysfunction in vitro and in vivo.21
In recent years, the use of PT in combination therapy has been investigated in several studies. PT reportedly sensitizes cancer cells to NSAIDs, anticancer drugs, and radiation.22-26 In addition, we previously showed that a combination of PT and 5-fluorouracil (5-FU) can overcome 5-FU resistance in human CRC cells.27 Although it has been reported that PT sensitizes TRAIL resistant hepatocellular carcinoma and breast cancer cells,10,28 it has not been examined whether combined treatment with PT can sensitizes TRAIL resistance in CRC cells.
In this study, we investigated whether PT sensitized CRC cells to TRAIL-induced apoptosis. We hypothesized that PT, in combination with TRAIL, would inhibit proliferation and induce apoptosis in CRC cells. Thus, our objective was to evaluate combination therapy with PT and TRAIL as a potential treatment for TRAIL-resistant CRC.
PT and z-VAD-FMK were purchased from Calbiochem (San Diego, CA, USA). TRAIL was purchased from Peprotech (Rocky Hill, NJ, USA). PT was dissolved in dimethylsulfoxide (DMSO; Sigma, St. Louis, MO, USA) to a concentration of 100 ┬ĄM and stored in the dark at -20Ōäā. Annexin-V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) were purchased from Invitrogen (Eugene, OR, USA). Hoechst 33258 was purchased from Sigma (St. Louis, MO, USA). Anti-Bcl2, anti-Bid, anti-Bax, anti-cytochrome C, anti-p53 and anti-caspase 3 antibodies were purchased from Santa Cruz Technology (Beverly, MA, USA). Anti-cFLIP and anti-caspase-9 antibodies were purchased from Cell Signaling (Beverly, MA, USA). Anti-actin antibody was purchased from Sigma.
The human CRC cell lines, HT-29 and HCT 116 cells (American Type Culture Collection, Rockville, MD, USA), were used as TRAIL-resistant and TRAIL-sensitive cells, respectively. The cells were cultured in RPMI 1640 medium supplemented with 10% FBS, 100 units of penicillin, and 100 units of streptomycin. For PT or TRAIL treatment, cells were sub-cultured in RPMI 1640 medium without FBS for 12 hours. PT and TRAIL were diluted with FBS-free medium to achieve desired concentrations. DMSO was diluted to the equivalent concentration and applied to the cells as a control.
Human CRC cells were plated at a density of 1.0├Ś104 cells per well in 96 well plates. Cells were treated with PT and/or TRAIL for 24 hours, before the medium was removed from each well and replaced with 200 ┬ĄL of fresh medium plus 20 ┬ĄL of 3-(4, 5-dimethylthiazol-2yl)-2, 5-diphenyltetrazolium bromide (MTT, 2.5 mg dissolved in 50 ┬ĄL of DMSO). After incubation for 4 hours at 37Ōäā, the culture medium containing MTT was removed and 200 ┬ĄL of DMSO was added. Plates were placed on a shaker until the crystals dissolved. Viable cells were detected by measuring absorbance at 570 nm, by using a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
After 24 hours of treatment, cells were trypsinized, collected, washed with ice-cold phosphate-buffered saline (PBS), suspended in a 500 ┬ĄL annexin V binding buffer containing 5 ┬ĄL of annexin V-FITC, and incubated for 15 minutes at room temperature in the dark. The fluorescence was measured using a BD LSR flow cytometer (BD Biosciences, San Jose, CA, USA) and processed with Cell Quest software for analysis.
Cell cycle phase and sub-G1 distribution were determined by staining DNA with PI (Sigma-Aldrich), a fluorescent biomolecule (Ex/Em=488 nm/617 nm). In brief, 1├Ś106 cells were incubated with one or both agents for 24 hours. Cells were then washed with PBS and fixed in 70% ethanol overnight. Cells were washed again with PBS and then incubated with PI (10 ┬Ąg/mL) and simultaneously treatment with RNase at 37Ōäā for 1 hour. The percentage of cells in different phases of the cell cycle or having sub-G1 DNA content was measured with a BD LSR flow cytometer and analyzed using Cell Quest software.
Apoptosis was identified by the presence of DNA condensation in Hoechst 33258-staining cells. The cells were treated for 24 hours, and then stained with Hoechst 33258 (1 ┬Ąg/mL) at 37Ōäā for 10 minutes. To identify cells undergoing apoptosis, nuclear morphology was examined using confocal laser scanning microscope (Carl Zeiss, Jena, Germany).
After 24 hours of treatment, cells were collected, washed twice with PBS, and then lysed for 30 minutes on ice, in lysis buffer (50 mM Tris-HCl pH 8.0, 150 mM EDTA, 1% TritonX-100, 0.5% sodium dodecyl sulfate [SDS] and protease inhibitor cocktail). The protein concentrations in the cell lysates were measured using the Protein Quantification kit from Bio-Rad. 50 ┬Ąg of total protein was loaded onto a SDS-PAGE gel. After transferring and blocking, the membrane was probed with the following antibodies (anti-Bcl2, anti-Bid, anti-Bax, anti-cytochrome C, anti-p53, anti-caspase-3, anti-caspase-9, anti-cFLIP and anti-actin). The signal was detected using enhanced Westone (Intron, Daejeon, Korea) and analyzed using the Las-3000 luminescent Image Analyzer (Fuji Film, Tokyo, Japan).
The data are presented as mean┬▒standard error (SE) of at least three independent experiments done in duplicate. Representative Western blots are shown. All the data was entered into Microsoft Excel 5.0, and SPSS Software was used to perform two-tailed t-tests or analysis of variance. P values<0.05 were considered significant.
The human colorectal cancer cell lines HT-29 and HCT-116 were treated with TRAIL at various concentrations (0, 5, 10, 25, 50 or 100 ng/mL). After 24 hours of treatment, cell viability was detected using the MTT assay. Treatment of HT-29 cells with TRAIL alone (100 ng/mL) decreased cell viability by approximately 15% (Fig. 1A). In contrast, treatment of HCT 116 cells with TRAIL (100 ng/mL) dramatically reduced viability in a dose-dependent manner, with cell showing a 70% decrease in viability. This indicates that HT-29 cells are highly resistant to TRAIL-induced cell death.
To determine the synergistic effect of PT on TRAIL-induced cell death, HT-29 cells were incubated in the absence or presence of PT (10 ┬ĄM) and TRAIL (5, 25, or 40 ng/mL). TRAIL alone did not inhibit cell survival (less than 10%), whereas combined treatment with PT exhibited a does dependent decrease in cell viability (Fig. 1B).
To support the earlier observations, annexin-V analysis was performed using FACScan. As shown in Fig. 2A, approximately 15.29% of HT-29 cells treated with PT were annexin V-positive, a value comparable to that of TRAIL-treated cells (8.6%). Co-treatment with TRAIL and PT caused a 3-fold increase in the proportion of annexin V-positive cells (41.86%), indicating that PT promotes TRAIL-induced apoptosis in TRAIL-resistant cells.
We also evaluated cell cycle modifications induced by PT and TRAIL in HT-29 cells. 24 hours after incubation with one or both agents, cells were analyzed by PI staining and flow cytometric analysis. Treating cells with PT and/or TRAIL resulted in the presence of a sub-G1 population, indicating apoptotic cell death. Peaks accounting for 11.34% and 8.07% of the overall cell population were detected in cells treated with PT or TRAIL, respectively. In combined treatment, a much greater sub-G1 population (27.77%) was observed, indicating that the combination of two agents dramatically promoted apoptosis in TRAIL-resistant cells (Fig. 2B).
Next, cells were stained with Hoechst 33258 and visualized by confocal microscopy to determine the presence apoptotic nuclear morphology. After treatment with either PT or TRAIL alone, cells were regular in morphology and formed confluent colonies, with cells rarely sloughing off. In contrast, upon treatment with both agents, HT-29 cells exhibited apoptotic characteristics, including cell shrinkage, nuclear condensation, and nuclear fragmentation. Moreover, the pan-caspase inhibitor Z-VAD-FMK blocked the nuclear fragmentation and condensation induced by the combination treatment, indicating that the change in nuclear morphology is mediated by the activation of caspase (Fig. 2C).
Many anticancer agents are capable of initiating caspase activation and inducing apoptotic cell death.29 The effect of PT, TRAIL, or PT plus TRAIL treatment on caspase activation in TRAIL resistant cells was examined. Western blot analysis revealed that the levels of full length caspase-3 and -9 in cells co-treated with PT plus TRAIL were significantly decreased compared to those in cells treated with PT or TRAIL alone. Furthermore, pretreatment with pan-casepase inhibitor Z-VAD-FMK significantly blocked the decrease in caspase-3 and -9 levels induced by co-treating cells with PT and TRAIL. These data indicate that co-treatment with PT and TRAIL activates caspases, promoting their enzymatic activity (Fig. 3A).
cFLIP inhibits the activation of caspase-8, the factor that initiates apoptosis.30 Thus, we investigated whether treatment with PT and TRAIL affected the levels of cFLIP proteins in HT-29 cells. Relative to the level of cFLIP detected in cells treated with either PT or TRAIL alone, the level of cFLIP decreased in response to combined treatment (Fig. 3B).
To evaluate the mechanisms responsible for apoptosis induced by the combination of TRAIL and PT, we investigated the expression level of several pro-apoptosis and anti-apoptosis proteins. In comparison to cells treated with PT or TRAIL alone, Western blot analysis showed that the level of Bcl-2 was significantly decreased by co-treatment. In contrast, the level of expression of truncated-Bid and Bax were significantly increased by combined treatment with PT and TRAIL when compared to those found in cells treated with PT or TRAIL alone (Fig. 4; first, second and third panel).
A consequence associated with decreased Bcl-2 levels is the dissipation of mitochondrial potential and the release of the mitochondrial pro-apoptotic protein, cytochrome C into the cytosol. After combined treatment with PT and TARIL, the release of cytochrome C was increased relative to control-treated cells and cells treated with PT or TRAIL alone (Fig. 4; fourth panel).
In addition, the p53 gene, a hallmark of apoptosis, is inactivated in the majority of human cancers.31 When compared to PT or TRAIL treatment alone, combination treatment significantly increased the level of p53 (Fig. 4; fifth panel). Taken together, these results indicate that the apoptosis induced by the combination of TRAIL and PT may be associated with the mitochondrial pathway.
Combination drug therapies have played a prominent role in cancer therapy. Exploration of the molecular mechanisms underlying the synergistic effects achieved by various chemotherapeutic agents would useful developing combination therapeutics that could significantly improve the prognosis of cancer patients.
In this study, we investigated the consequences of TRAIL and PT combination treatment. This treatment resulted in increased apoptosis and caspase-activation, and enhanced TRAIL sensitivity in TRAIL-resistant CRC cells. Therefore, the combination of TRAIL and PT might overcome TRAIL resistance in CRC cells and be an effective therapeutic strategy for patients with CRC.
Two previous studies have demonstrated that PT sensitizes cells to TRAIL. In breast cancer cells, it has been shown that PT attenuates resistance to TRAIL-induced apoptosis.28 PT was also found to sensitize hepatocellular carcinoma cells to TRAIL-induced apoptosis.10 Based on these studies, we hypothesized that PT is a promising candidate for combination therapy with TRAIL in CRC. To support our hypothesis, we first examined the sensitivity of human CRC cell lines to TRAIL. As shown in Fig. 1A, TRAIL inhibited proliferation of HCT 116 cells in a dose-dependent manner; notably, however, this reduction did not occur in TRAIL-resistant HT-29 cells, even at higher doses of TRAIL. These results confirmed that HT-29 cells are resistant to TRAIL and are consistent with the results of previous studies exploring the sensitivity of human CRC cells to TRAIL treatment.32,33 Interestingly, the combined treatment with PT and TRAIL reduced cell proliferation and increased apoptotic cell death in TRAIL-resistant HT-29 cells.
Many factors for apoptosis converge to activate caspase-3-the key protease and final effector in the apoptosis pathway.34 The caspase-dependent apoptosis pathway includes the mitochondrial, death receptor, and endoplasmic reticulum (ER) pathways.35,36 The mitochondrial pathway is regulated by the Bcl-2 family, which is divided into two groups: the anti-apoptotic members (Bcl-2, Bcl-xl)36,37 and pro-apoptotic members (Bax, Bad, Bid).38 In this study, combined PT and TRAIL treatment altered the levels of Bcl-2, truncated-Bid, Bax, caspase-9, and caspase-3. Moreover, the decrease of both caspase-9 and -3 was prevented by pretreatment with a pan-caspase inhibitor, Z-VAD-FMK. These results demonstrate that combined PT and TRAIL treatment circumvent TRAIL resistance in CRC cells. Furthermore, these data suggest that apoptosis induced by combined PT and TRAIL treatment is under the control of a caspase- and mitochondrial pathway.
The canonical tumor suppressor gene, p53, mediates G1 growth arrest by inducing the cyclin-dependent kinase inhibitor p21, and participates in apoptosis through transactivation of the pro-apoptotic Bax gene in response to DNA-damage.39,40 The p53 gene, which is inactivated in a majority of human cancers,31 has been proposed as an accurate indicator that CRC is responding to anticancer drugs. In this study, we demonstrated that the level of p53 was enhanced by combined PT and TRAIL treatment.
A structural homologue of caspase-8, the anti-apoptotic factor cFLIP binds to FADD, competing with caspase-8 for recruitment to DISC. The overexpression of cFLIP in tumor cells has been shown to determine the resistance of a tumor to TRAIL.41,42 Importantly, aberrant up-regulation of cFLIP proteins is present in a number of cancers. In addition, down-regulation of cFLIP by small interfering RNA is sufficient to sensitize TRAIL-resistant tumor cell lines to TRAIL-induced apoptosis.43,44 Although we did not show the change in caspase-8 expression, the downregulation of cFLIP was observed in HT-29 cells under combined treatment conditions, implicating the involvement of reduced cFLIP levels in this process.
In summary, we investigated whether combined PT and TRAIL treatment could inhibit cell proliferation and induce apoptotic cell death in TRAIL-resistant CRC cells. Combined treatment with PT and TRAIL upregulates pro-apoptotic proteins and downregulates anti-apoptotic proteins, leading to the release of cytochrome C, increased caspase activation, and subsequently, increased apoptosis. Taken together, these results suggest that PT sensitizes cells to TRAIL via mitochondrial- and caspase-dependent apoptotic pathways in human colorectal cancer cells. For this reason we believe that combined treatment with PT and TRAIL could represent a new and important therapeutic strategy for CRC treatment.
NOTES
References
1. Gonzalvez F, Ashkenazi A. New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene 2010;29:4752-4765.PMID: 20531300.


2. Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer 2008;8:782-798.PMID: 18813321.


3. Shi YG. Mechanisms of caspase activation and inhibition during apoptosis. Molecular Cell 2002;9:459-470.PMID: 11931755.


4. Koschny R, Walczak H, Ganten TM. The promise of TRAIL - potential and risks of a novel anticancer therapy. J Mol Med (Berl) 2007;85:923-935.PMID: 17437073.


5. Jin Z, McDonald ER, Dicker DT, El-Deiry WS. Deficient tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor transport to the cell surface in human colon cancer cells selected for resistance to TRAIL-induced apoptosis. J Biol Chem 2004;279:35829-35839.PMID: 15155747.


6. Kim HS, Lee JW, Soung YH, et al. Inactivating mutations of caspase-8 gene in colorectal carcinomas. Gastroenterology 2003;125:708-715.PMID: 12949717.


7. Hernandez A, Wang QD, Schwartz SA, Evers BM. Sensitization of human colon cancer cells to TRAIL-mediated apoptosis. J Gastrointest Surg 2001;5:56-65.PMID: 11309649.


8. Burns TF, El-Deiry WS. Identification of inhibitors of TRAIL-induced death (ITIDs) in the TRAIL-sensitive colon carcinoma cell line SW480 using a genetic approach. J Biol Chem 2001;276:37879-37886.PMID: 11486001.


9. Cummins JM, Kohli M, Rago C, Kinzler KW, Vogelstein B, Bunz F. X-linked inhibitor of apoptosis protein (XIAP) is a nonredundant modulator of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis in human cancer cells. Cancer Res 2004;64:3006-3008.PMID: 15126334.


10. Carlisi D, D'Anneo A, Angileri L, et al. Parthenolide sensitizes hepatocellular carcinoma cells to TRAIL by inducing the expression of death receptors through inhibition of STAT3 activation. J Cell Physiol 2011;226:1632-1641.PMID: 21413021.


11. Seo OW, Kim JH, Lee KS, et al. Kurarinone promotes TRAIL-induced apoptosis by inhibiting NF-kappaB-dependent cFLIP expression in HeLa cells. Exp Mol Med 2012;44:653-664.PMID: 22932446.



12. Kauntz H, Bousserouel S, Gosse F, Raul F. The flavonolignan silibinin potentiates TRAIL-induced apoptosis in human colon adenocarcinoma and in derived TRAIL-resistant metastatic cells. Apoptosis 2012;17:797-809.PMID: 22555452.


13. Knight DW. Feverfew: chemistry and biological activity. Nat Prod Rep 1995;12:271-276.PMID: 7792073.


14. Sohma I, Fujiwara Y, Sugita Y, et al. Parthenolide, an NF-kappaB inhibitor, suppresses tumor growth and enhances response to chemotherapy in gastric cancer. Cancer Genomics Proteomics 2011;8:39-47.PMID: 21289336.

15. Zhao LJ, Xu YH, Li Y. Effect of parthenolide on proliferation and apoptosis in gastric cancer cell line SGC7901. J Dig Dis 2009;10:172-180.PMID: 19659784.


16. Park JH, Liu L, Kim IH, Kim JH, You KR, Kim DG. Identification of the genes involved in enhanced fenretinide-induced apoptosis by parthenolide in human hepatoma cells. Cancer Res 2005;65:2804-2814.PMID: 15805281.


17. Zanotto-Filho A, Braganhol E, Schroder R, et al. NFkappaB inhibitors induce cell death in glioblastomas. Biochem Pharmacol 2011;81:412-424.PMID: 21040711.


18. Dai Y, Guzman ML, Chen S, et al. The NF (Nuclear factor)-kappaB inhibitor parthenolide interacts with histone deacetylase inhibitors to induce MKK7/JNK1-dependent apoptosis in human acute myeloid leukaemia cells. Br J Haematol 2010;151:70-83.PMID: 20701602.



19. Sun Y, St Clair DK, Xu Y, Crooks PA, St Clair WH. A NADPH oxidase-dependent redox signaling pathway mediates the selective radiosensitization effect of parthenolide in prostate cancer cells. Cancer Res 2010;70:2880-2890.PMID: 20233868.



20. Zhang S, Ong CN, Shen HM. Involvement of proapoptotic Bcl-2 family members in parthenolide-induced mitochondrial dysfunction and apoptosis. Cancer Lett 2004;211:175-188.PMID: 15219941.


21. Kim SL, Trang KT, Kim SH, et al. Parthenolide suppresses tumor growth in a xenograft model of colorectal cancer cells by inducing mitochondrial dysfunction and apoptosis. Int J Oncol 2012;41:1547-1553.PMID: 22895542.


22. Fang LJ, Shao XT, Wang S, Lu GH, Xu T, Zhou JY. Sesquiterpene lactone parthenolide markedly enhances sensitivity of human A549 cells to low-dose oxaliplatin via inhibition of NF-kappaB activation and induction of apoptosis. Planta Med 2010;76:258-264.PMID: 19774508.


23. Gao ZW, Zhang DL, Guo CB. Paclitaxel efficacy is increased by parthenolide via nuclear factor-kappaB pathways in in vitro and in vivo human non-small cell lung cancer models. Curr Cancer Drug Targets 2010;10:705-715.PMID: 20578985.


24. Yun BR, Lee MJ, Kim JH, Kim IH, Yu GR, Kim DG. Enhancement of parthenolide-induced apoptosis by a PKC-alpha inhibition through heme oxygenase-1 blockage in cholangiocarcinoma cells. Exp Mol Med 2010;42:787-797.PMID: 20938215.



25. Lee CS, Kim YJ, Lee SA, Myung SC, Kim W. Combined effect of Hsp90 inhibitor geldanamycin and parthenolide via reactive oxygen species-mediated apoptotic process on epithelial ovarian cancer cells. Basic Clin Pharmacol Toxicol 2012;111:173-181.PMID: 22433057.


26. Yip-Schneider MT, Nakshatri H, Sweeney CJ, Marshall MS, Wiebke EA, Schmidt CM. Parthenolide and sulindac cooperate to mediate growth suppression and inhibit the nuclear factor-kappa B pathway in pancreatic carcinoma cells. Mol Cancer Ther 2005;4:587-594.PMID: 15827332.


27. Kim SL, Kim SH, Trang KT, et al. Synergistic antitumor effect of 5-fluorouracil in combination with parthenolide in human colorectal cancer. Cancer Lett 2013;335:479-486.PMID: 23507557.


28. Nakshatri H, Rice SE, Bhat-Nakshatri P. Antitumor agent parthenolide reverses resistance of breast cancer cells to tumor necrosis factor-related apoptosis-inducing ligand through sustained activation of c-Jun N-terminal kinase. Oncogene 2004;23:7330-7344.PMID: 15286701.


29. Fulda S, Debatin KM. Death receptor signaling in cancer therapy. Curr Med Chem Anticancer Agents 2003;3:253-262.PMID: 12769771.


30. Poukkula M, Kaunisto A, Hietakangas V, et al. Rapid turnover of c-FLIPshort is determined by its unique C-terminal tail. J Biol Chem 2005;280:27345-27355.PMID: 15886205.


31. Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990;61:759-767.PMID: 2188735.


32. Lee SC, Cheong HJ, Kim SJ, et al. Low-Dose Combinations of LBH589 and TRAIL Can Overcome TRAIL-resistance in Colon Cancer Cell Lines. Anticancer Res 2011;31:3385-3394.PMID: 21965751.

33. Park MH, Jo M, Won D, Song HS, Song MJ, Hong JT. Snake venom toxin from Vipera lebetina turanica sensitizes cancer cells to TRAIL through ROS- and JNK-mediated upregulation of death receptors and downregulation of survival proteins. Apoptosis 2012;17:1316-1326.PMID: 23007278.


34. Fernandes-Alnemri T, Litwack G, Alnemri ES. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem 1994;269:30761-30764.PMID: 7983002.


36. Yang E, Korsmeyer SJ. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood 1996;88:386-401.PMID: 8695785.


37. Mignotte B, Vayssiere JL. Mitochondria and apoptosis. Eur J Biochem 1998;252:1-15.PMID: 9523706.


38. Reed JC. Double identity for proteins of the Bcl-2 family. Nature 1997;387:773-776.PMID: 9194558.



39. Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 1995;80:293-299.PMID: 7834749.


40. Sionov RV, Haupt Y. The cellular response to p53: the decision between life and death. Oncogene 1999;18:6145-6157.PMID: 10557106.



41. Ullenhag GJ, Mukherjee A, Watson NF, Al-Attar AH, Scholefield JH, Durrant LG. Overexpression of FLIPL is an independent marker of poor prognosis in colorectal cancer patients. Clin Cancer Res 2007;13:5070-5075.PMID: 17785559.


42. Wang X, Chen W, Zeng W, et al. Akt-mediated eminent expression of c-FLIP and Mcl-1 confers acquired resistance to TRAIL-induced cytotoxicity to lung cancer cells. Mol Cancer Ther 2008;7:1156-1163.PMID: 18483303.



43. Dutton A, O'Neil JD, Milner AE, et al. Expression of the cellular FLICE-inhibitory protein (c-FLIP) protects Hodgkin's lymphoma cells from autonomous Fas-mediated death. Proc Natl Acad Sci U S A 2004;101:6611-6616.PMID: 15096587.



44. Mathas S, Lietz A, Anagnostopoulos L, et al. c-FLIP mediates resistance of Hodgkin/Reed-Sternberg cells to death receptor-induced apoptosis. J Exp Med 2004;199:1041-1052.PMID: 15078899.



Fig.┬Ā1
The inhibitory effect of combined parthenolide (PT) and tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) treatment on cell proliferation. (A) HT-29 and HCT 116 cells were treated with TRAIL for 24 hours, at the concentrations indicated, before being analyzed for viability by 3-(4, 5-dimethylthiazol-2yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Data represent the mean┬▒SE of at least three independent experiments. (B) Cells were co-treated with PT (10 ┬ĄM) and TRAIL (25 ng/mL) for 24 hours. The data represent the mean┬▒SE of three independent experiments. *P<0.05, compared to control.
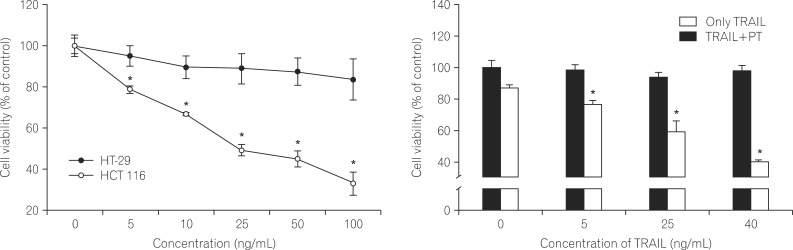
Fig.┬Ā2
The apoptotic effect of combined parthenolide (PT) and tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) treatment. (A) Apoptotic cell death induced by combination treatment. After treatment with TRAIL and/or 5-fluorouracil (5-FU) for 24 hours, cells were harvested and stained with annexin V-fluorescein isothiocyanate (FITC), to show that apoptosis is induced by combination treatment. (B) Cell cycle modification induced by combination treatment. After treatment with PT and/or TRAIL for 24 hours, cells were harvested and stained with propidium iodide (PI). The percentage of sub-G1 cells within the population is shown in each histogram. The total number of cells analyzed for each condition was 10,000. (C) DNA condensation and fragmentation induced by combination treatment. Apoptosis-associated DNA condensation was examined using Hoechst 33258 (1 ┬Ąg/mL) staining. Apoptotic nuclei show intense fluorescence, corresponding to chromatin condensation (arrowhead) and fragmentation.

Fig.┬Ā3
Regulation of caspase and converting enzyme-inhibitory protein (cFLIP) levels by treatment with parthenolide (PT) and/or tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL). (A) Cell lysates were prepared after treatment for 24 hours and then analyzed by western blot. The levels of full-length caspase-3 and -9 proteins were decreased by combination treatment. Caspase-3 and -9 levels, however, were not altered when cells were pre-treated with the pan-caspase inhibitor Z-VAD-FMK. Actin was used as a loading control. (B) The protein level of cFLIP was decreased by combination treatment. Actin was used as a loading control.
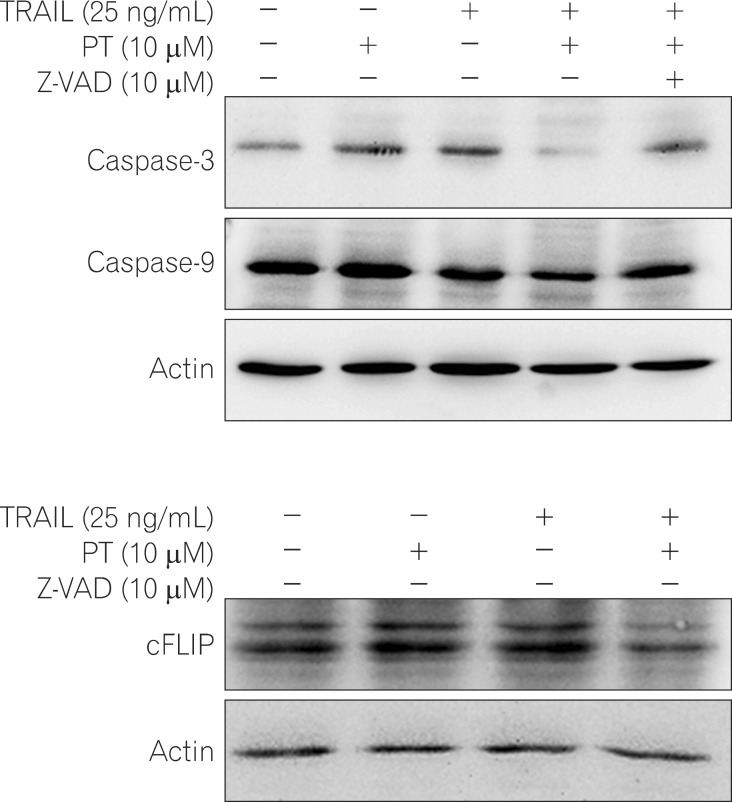
Fig.┬Ā4
The effect of combined parthenolide (PT) and tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) treatment on apoptosis-associated protein expression. Cell lysates were prepared after treatment for 24 hours and analyzed by western blots. The following proteins were probed: B-cell lymphoma 2 (Bcl-2), Bid, Bax, cytochrome C, and p53. The level of the anti-apoptotic protein Bcl-2 was significantly decreased by combined treatment with PT and TRAIL. Conversely, pro-apoptotic proteins, cleaved-Bid and Bax, were increased under combined treatment conditions. The levels of cytochrome C and p53 were increased after combination treatment. Actin was used as a loading control.







